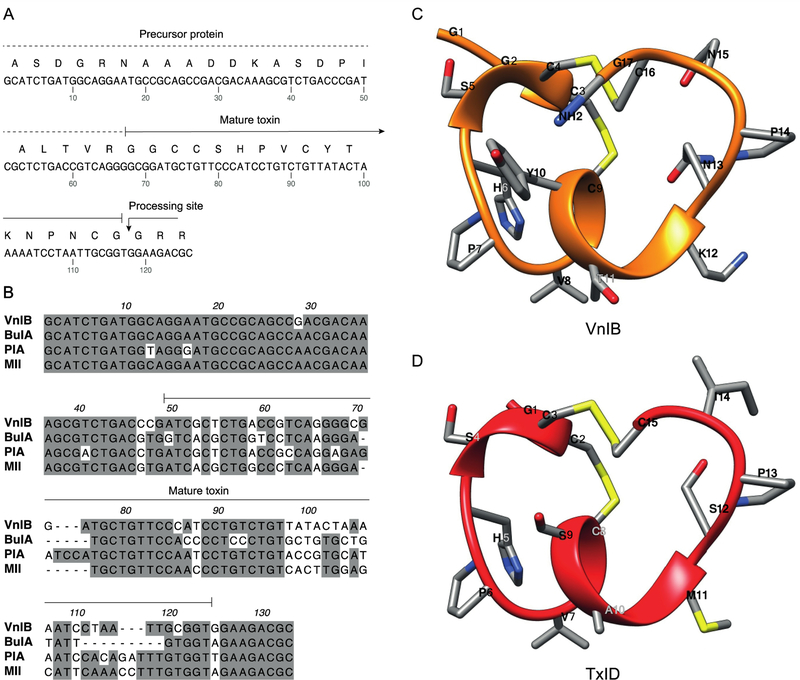
Figure 1. α-Conotoxin (α-Ctx) VnIB.
(A) The α-Ctx VnIB pro-peptide and the encoded conopeptide sequence is shown (GenBank accession number MK120500). The precursor protein is indicated by a dotted line and the mature toxin by a solid line. The presumed C-terminal amide processing site at the terminal Gly residue is indicated by an arrow. (B) Alignment of the nucleic acid sequences of α-Ctx VnIB, BuIA, PIA and MII, which all exhibit potent antagonistic activity at α6-containing (α6*) nicotinic acetylcholine receptors (nAChRs). Conserved residues are shaded in grey. Alignments were made using MacVector. (C-D) Ribbon representation of lowest energy structures of VnIB (orange) and TxID (red) with stick representations of amino acid side chains. The structural representation of VnIB was generated in USCF Chimera based on molecular-dynamics simulations of TxID in the α6β4 binding site published by J. Yu et al. (Pettersen et al., 2004; Yu et al., 2018).