Table 6. General dose modification guidelines for lorlatinib‐related adverse reactions by CTCAE gradea .
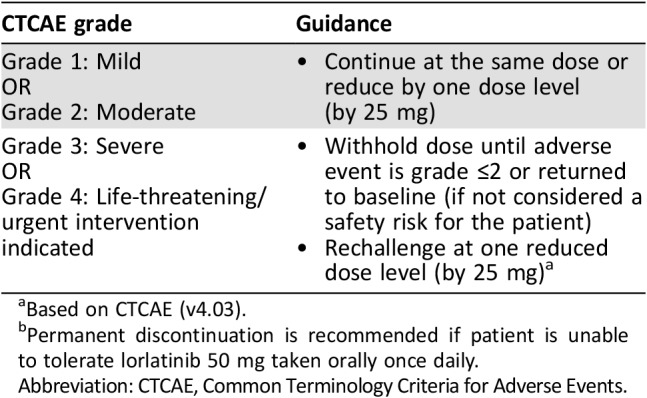
Based on CTCAE (v4.03).
Permanent discontinuation is recommended if patient is unable to tolerate lorlatinib 50 mg taken orally once daily.
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
