Figure 4.
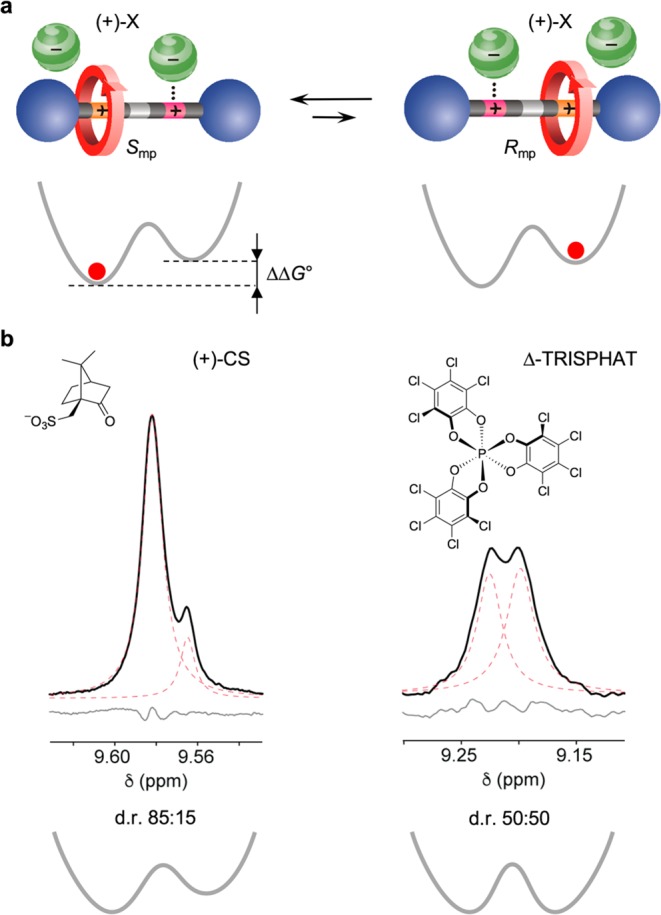
(a) Interconversion between two diastereomeric ion pairs composed of a coconformationally MP chiral rotaxane dication, such as 12+, and a chiral monoanion. In the proposed structures, one anion is coordinated to the unencircled triazolium, while another is weakly paired with the encircled site. Simplified potential energy curves for the location of the ring along the axle are also shown. As the two ion pairs can have different stabilities [ΔΔG° ≠ 0], the ring distribution between the two identical stations can become unbalanced. (b) Partial 1H NMR spectra (500 MHz) of the HTruresonance in 12+ after the addition of 8 equiv of the tetrabutylammonium salt of (1S)-(+)-10-camphorsulfonate (CD2Cl2, 223 K; left) or Δ-TRISPHAT (toluene-d8, 243 K; right). Black, red, and gray traces show respectively the experimental spectrum, the deconvoluted peaks, and the fitting residuals.
