Fig. 1. Protein dynamics into the hundreds of nanosecond to microsecond range accessible to NP-assisted solution NMR.
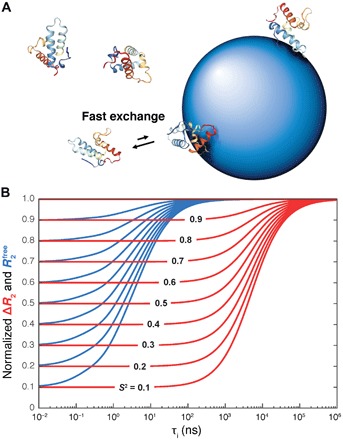
(A) Protein molecules are in fast exchange between their rapidly tumbling free state and a slowly tumbling NP-bound state giving rise to effective transverse spin relaxation rates versus in the absence of NPs. (B) Simulated dependence of in the absence of NPs (blue) and in the presence and absence of NPs (red) on the internal correlation time τi and motional restriction (S2 order parameter), which demonstrates the wide range of time scales sensitively probed by ΔR2. The blue and red curves were normalized by setting their maximal values to 1.0.
