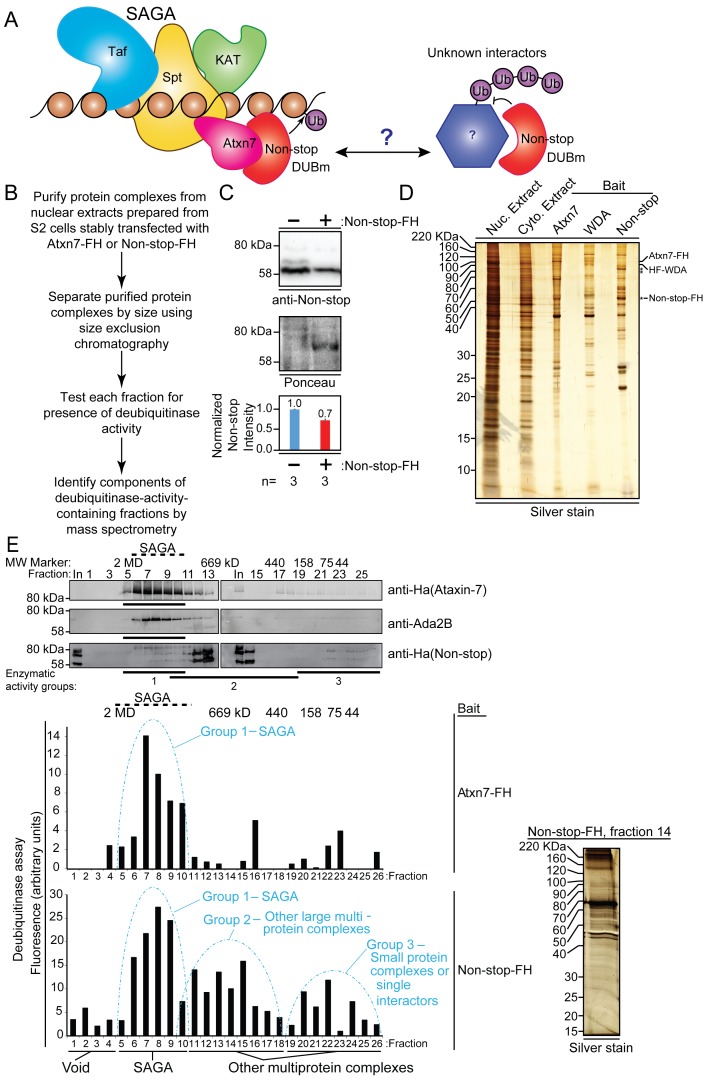
Figure 1. Non-stop (Not) co-purifies with Spt Ada Gcn5 acetyltransferase (SAGA) complex and additional multi-protein complexes.
(A) Working model: The Non-stop-containing SAGA deubiquitinase module (DUBm) functions distally from SAGA. Non-stop is anchored to SAGA by Ataxin-7 (Atxn7) but is released to interact with unknown partners. (B) Scheme to identify Non-stop interactors. (C) Characterization of Non-stop-2xFLAG-2xHA (FH)-expressing S2 cell lines. Stably transfected cells were treated with 10 uM copper sulphate show comparable levels of Non-stop expression in parental and stably transfected cell lines. Parental S2 cells containing no plasmid compared to S2 cells stably transfected with Non-stop plasmid. Quantitation of anti-Non-stop immunoblot signal intensities normalized to Ponceau total protein loading control. Error bars represent standard error. (D) Analysis of purified complexes. Non-stop-containing complexes were analyzed by silver staining. Atxn7 and Will Decrease Acetylation (WDA)-containing complexes are shown for comparison. (E) Protein complexes, purified and fractionated as described in B were analyzed by immunoblotting to observe relative elution by size (top, Atxn7 and Ada2B recreated from Mohan et al., 2014b) followed by measure of deubiquitinase activity contained in each fraction as assayed by ubiquitin-AMC assay in which increased fluorescence correlates directly with deubiquitination activity. The complexity of eluted fractions was analyzed and Group 2 peak fraction, number 14, is shown (right).
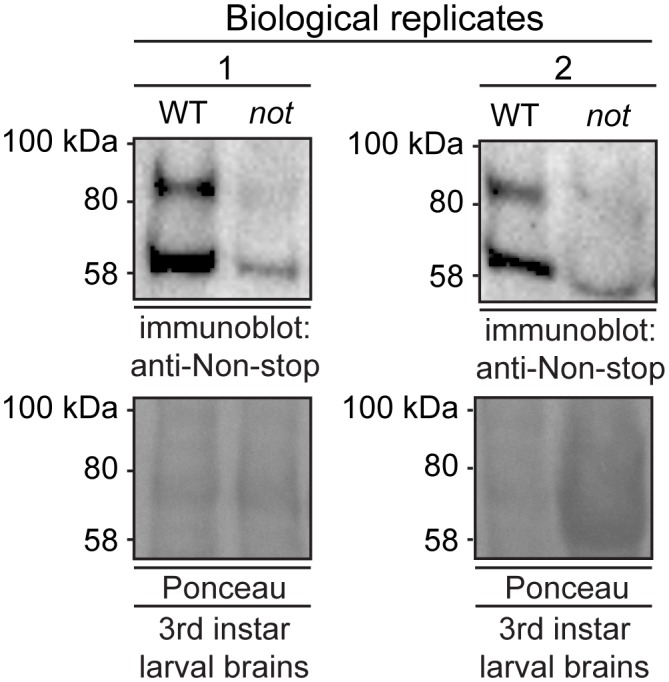
Figure 1—figure supplement 1. Anti-Non-stop antibody specifically recognizes Non-stop.