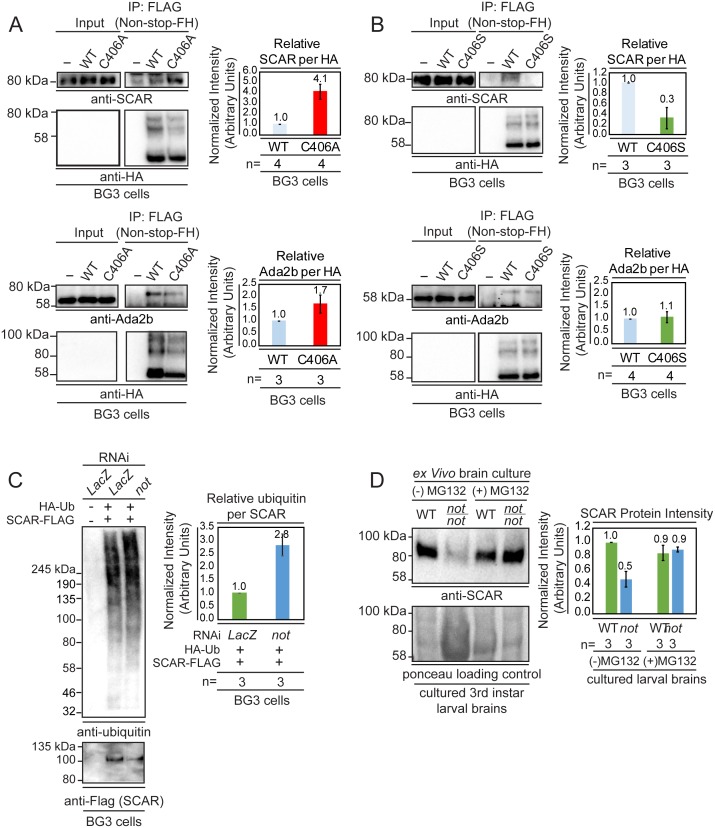
Figure 4. Non-stop binds ubiquitinated SCAR, regulates SCAR ubiquitination, and entry into the proteasome degradation pathway.
(A) Non-stop catalytic mutation which increases substrate binding also increases interaction with SCAR. The Non-stop catalytic cysteine was mutated to alanine creating Non-stop C406A-2xFLAG-2xHA (C406A). The wild type version of Non-stop (WT), the mutant version of Non-stop (C406A), or no plasmid (-) were transfected into BG3 cells and whole cell extracts were prepared. Flag resin was used to immunoprecipitate Non-stop-FH protein. Extracts were immunoblotted for SCAR. Immunoblots for Ada2b control for incorporation into the SAGA complex. Immunoblots for HA verify the presence of the Non-stop-FH constructs. Ada2b and SCAR intensity were quantified and normalized to HA. Values are shown as relative to WT. Error bars represent standard error. (B) Non-stop catalytic mutation which decreases substrate binding also decreases interaction with SCAR. The Non-stop catalytic cysteine was mutated to serine (C406S). BG3 cells were mock transfected (-), transfected with Non-stop-FH, or with Non-stop C406S-FH (C406S) and whole cell extracts were prepared. Flag resin was used to immunoprecipitate the Non-stop-FH constructs. Pull-downs were immunoblotted for SCAR. Incorporation into SAGA was verified by Ada2b immunoblot. HA immunoblots verify the presence of the Non-stop-FH constructs. Ada2b and SCAR intensity was quantified and normalized to HA. Values are shown as relative to wild type. Error bars are standard error. (C) Non-stop counters polyubiquitination of SCAR. BG3 cells were treated with dsRNA targeting either non-stop or LacZ and transfected with HA-ubiquitin (HA-Ub) and SCAR-FLAG (SCAR-F). Control cells were treated with LacZ dsRNA and a mock transfection was performed. After six days, cells were treated with MG132 protease inhibitor for 6 hr and denaturing whole cell extracts were made. Anti-flag resin was used to capture SCAR-FLAG. Immunoblots were performed for ubiquitin (VU-1) and Flag to verify presence of SCAR. Protein intensity for VU-1 was measured and normalized to Flag protein intensity. Values are shown as relative to LacZ. Error bars represent standard error. (D) Non-stop counters proteasomal degradation of SCAR. WT or not brains were dissected from 3rd instar larva and cultured ex vivo. Half of the brains from each genotype were treated for 24 hr with protease inhibitor [50 µm MG132 (MG132+)] while the remaining brains served as untreated control (MG132-). Whole cell extracts were immunoblotted for SCAR. SCAR protein intensity was measured and normalized to Ponceau total protein control. Numbers are expressed as relative to WT untreated control. Error bars represent standard error.