Abstract
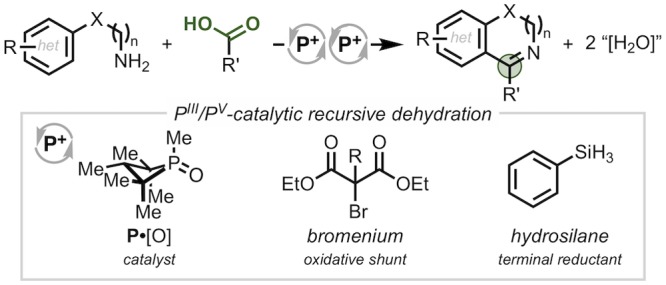
A method for the annulation of amines and carboxylic acids to form pharmaceutically relevant azaheterocycles via organophosphorus PIII/PV redox catalysis is reported. The method employs a phosphetane catalyst together with a mild bromenium oxidant and terminal hydrosilane reductant to drive successive C–N and C–C bond-forming dehydration events via the serial action of a catalytic bromophosphonium intermediate. These results demonstrate the capacity of PIII/PV redox catalysis to enable iterative redox-neutral transformations in complement to the common reductive driving force of the PIII/PV couple.
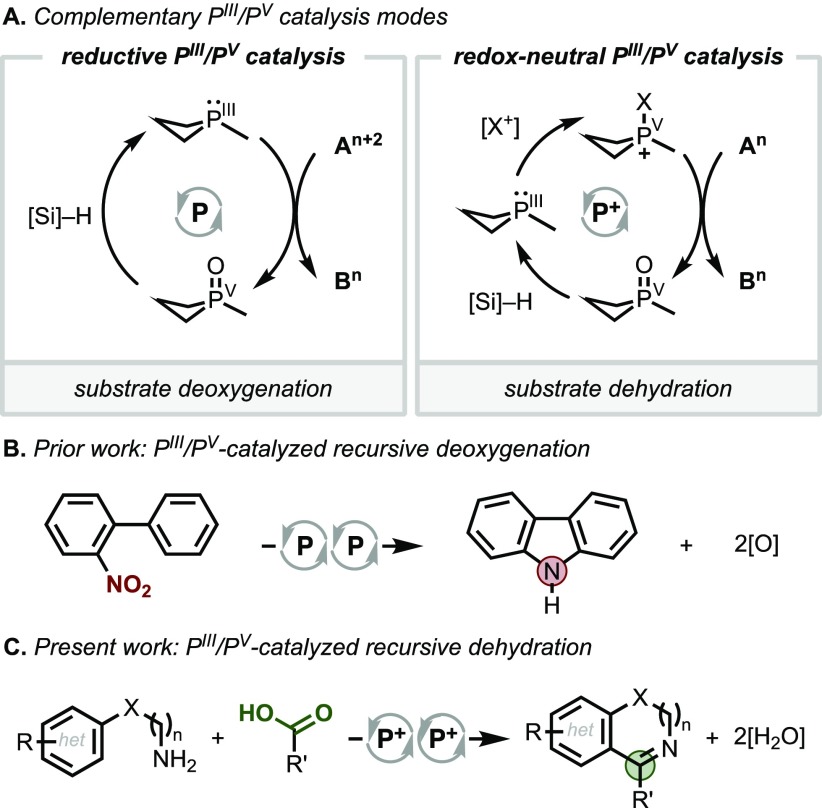
Progress over the past decade has established the viability of the PIII/PV=O redox couple for catalysis.1−3 In contrast to prior notions about the kinetic inertness of the P=O bond, the incorporation of P into a ring structure can lead to swift deoxygenation by mild reagents such as hydrosilanes.4,5 By virtue of the reducing potential of the PIII/PV couple, many of these transformations are reductive in nature (Figure 1A, left).6−8 In this vein, we have shown that four-membered ring organophosphorus catalysts effect reductive conversion of nitro9 and sulfonyl10 substrates (cf. Figure 1B) in which the ability to recursively renew a reactive PIII species under conditions of PIII/PV catalysis enables a single catalyst to perform successive deoxygenative operations on a substrate (i.e., autotandem catalysis11).
Figure 1.
(A) Complementary reductive and redox-neutral modes of PIII/PV catalysis. (B) Catalytic heterocyclization of nitrobiaryl by recursive deoxygenation. (C) Catalytic annulation of amines and carboxylic acids by recursive dehydration.
In addition to reductive chemistry, the versatile PIII/PV driving force can be adapted to achieve net redox-neutral transformations when paired with an appropriate oxidant as evoked in Mukaiyama’s conceptualization of an “oxidation-reduction condensation.”12 Within a PIII/PV-catalytic context,13 the introduction of a mild chemoselective halenium oxidant, for instance, can shunt the reductive manifold into a net redox-neutral mode, where the key reactive catalytic intermediate is not a phosphine but rather a halophosphonium cation14,15 (Figure 1A, right). Indeed, halophosphonium intermediates have been invoked by Rutjes and van Delft5 and Mecinović16 in the context of PIII/PV-catalyzed Appel halogenation and N-acylation reactions, respectively.17 In view of the fact that phosphonium reagents have been described as having “virtually ideal properties as selective oxygen extractors for net dehydration reactions,”18,19 the potential to achieve recursive dehydrations in an autotandem catalytic manner via a net redox-neutral mode of PIII/PV catalysis could be expected to present new opportunities for serial bond formation.
We show here an annulation of amines and carboxylic acids via recursive dehydration driven by a redox-active organophosphorus catalyst cycling in the PIII/PV couple (Figure 1C). This autotandem catalytic system enables the elaboration of simple starting materials into pharmaceutically relevant azaheterocycles through a condensation/cyclodehydration sequence in a one-pot catalytic protocol. The success of the approach relies on the mutual compatibility and functional interplay of the reducing and oxidizing reagents with the organophosphorus catalyst to orchestrate a sequence of distinct C–N and C–C bond-forming events. The ability of PIII/PV redox catalysis to encompass such recursive dehydration stands as a complement to existing deoxygenation methods, thus broadening the scope of transformations accessible to this catalytic mode.
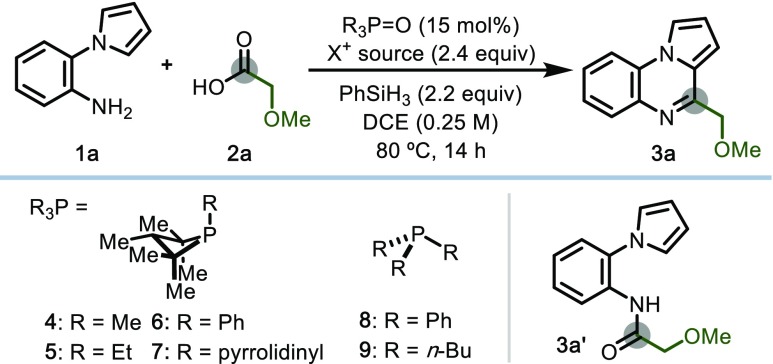
To evaluate the possibility of recursive dehydration driven by PIII/PV redox cycling, the tandem amidation/cyclodehydration of amine 1a and carboxylic acid 2a to generate pyrroloquinoxaline 3a was evaluated (Table 1). Optimal conditions using 1,2,2,3,4,4-hexamethylphosphetane P-oxide 4·[O]20 as catalyst, diethyl bromomalonate (DEBM) as oxidant, and phenylsilane as terminal reductant yielded the desired product in 94% yield, isolable on 0.4 mmol scale in 84% yield (Table 1, entry 1). A mild, weakly oxidizing bromenium reagent was found to be essential for the redox compatibility of the system, as demonstrated by Rutjes and van Delft;5 the related diethyl (methyl)bromomalonate (DEMBM) was similarly competent in the transformation (entry 2), but the more strongly oxidizing N-bromosuccinimide resulted in poor conversion to product (entry 3). Chlorenium oxidants such as diethyl chloromalonate (DECM) and carbon tetrachloride gave no dehydrative heterocyclization (entries 4 and 5); instead, amide 3a′ was obtained in 70% yield, indicating chlorophosphonium ion competency in C–N forming amidation but not C–C forming cyclodehydration.21
Table 1. Discovery and Optimization of Phosphacatalytic Iterative Condensation/Annulation of Amine and Carboxylic Acid.
| entry | R3P=O | X+ source | yielda (%) |
|---|---|---|---|
| 1 | 4·[O] | DEBM | 94 (84)b |
| 2 | 4·[O] | DEMBM | 90 |
| 3 | 4·[O] | NBS | 10 |
| 4 | 4·[O] | DECM | 0 |
| 5 | 4· [O] | CCl4 | 0 |
| 6 | 5·[O] | DEBM | 26 |
| 7 | 6·[O] | DEBM | 46 |
| 8 | 7·[O] | DEBM | 50 |
| 9 | 8·[O] or 9·[O] | DEBM | 0 |
| 10 | none | DEBM | 0 |
| 11 | 4·[O] | none | 0 |
| 12 | 4·[O], no PhSiH3 | DEBM | 0 |
| 13 | 4 | DEBM | 90 |
| 14 | [4·Br]Br | DEBM | 87 |
Yields determined through 1H NMR analysis with the aid of an internal standard. See Supporting Information for full synthetic details and yields of intermediate amide 3a′.
Isolated yield on 0.4 mmol scale. DCE = 1,2-dichloroethane; DEBM = diethyl bromomalonate; DEMBM = diethyl (methyl)bromomalonate; NBS = N-bromosuccinimide; DECM = diethyl chloromalonate.
With respect to catalyst, variation of the phosphetane exocyclic moiety to ethyl (5·[O], entry 6), phenyl (6·[O], entry 7), or pyrrolidino (7·[O], entry 8) all resulted in substantial decrease in the efficiency of the reaction, while acylic phosphine oxides (8·[O] or 9·[O], entry 9) fail to promote the cyclocondensation (see Supporting Information). Control experiments confirm that no azaheterocycle product is observed in the absence of any of 4·[O], phenylsilane, or DEBM (entries 10–12).22 Furthermore, employing PIII species 4 or pregenerated bromophosphonium [4·Br]Br23 in place of phosphine oxide 4·[O] resulted in comparable efficiency, consistent with the notion of PIII/PV=O redox cycling (entries 13 and 14).
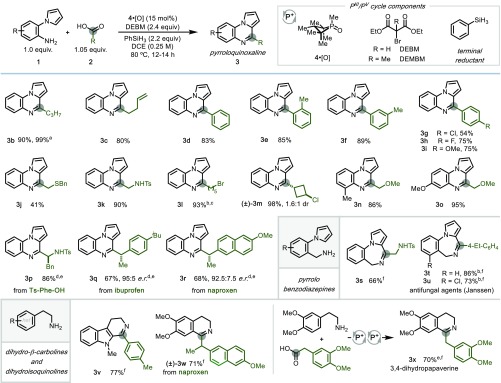
The optimized phosphacatalytic protocol provides direct access to complex azaheterocycles from simple and readily available amine and carboxylic acid starting materials (Figure 2). A variety of carboxylic acids are efficiently incorporated into pyrroloquinoxalines, including those possessing olefinic and aryl functionalities (3c–3i, 54–89% yields). This protocol was also readily translated to larger-scale reactions, as a 5 mmol scale reaction of 1-(2-aminophenyl)pyrrole and butyric acid provided 1.04 g of compound 3b in 99% yield with 8 mol % loading of organophosphorus catalyst 4·[O]. As demonstrated by products 3d–3i, the reaction efficiency is relatively independent of substitution on benzoic acid coupling partners, including changing steric profile (54–89% yields). Critically, acids containing polar functionalities, including alkyl ethers, thioethers, sulfonamides, and alkyl halides, undergo efficient iterative dehydration to provide heterocycles in good to excellent yields (3a, 3j–3p, 41–90% yields). Of particular note are the amino acid-incorporating products 3k and 3p, which originate from protected Ts-Gly-OH and Ts-Phe-OH. Further, both primary and secondary haloalkane functionalities are conserved under this PIII/PV-catalytic manifold (3l and 3m, 93% and 98% yields, respectively). Substitution on the aniline ring was well-tolerated; both ortho- and meta-substituted pyrroloanilines were readily incorporated into heterocyclic scaffolds (3n and 3o, 86% and 95% yields, respectively). Chiral carboxylic acids, such as ibuprofen and naproxen, are incorporated with good yield and high stereochemical fidelity (3q, 67%, 95:5 enantiomeric ratio (e.r.); 3r, 68%, 92.5:7.5 e.r.) under modified recursive dehydration conditions (precatalyst [4·Br]Br, MeCN, 50 °C; see Supporting Information for full synthetic details).
Figure 2.
Examples of autotandem phosphacatalytic annulation of amines and carboxylic acids. All yields isolated on 0.4 mmol scale unless indicated otherwise. See Supporting Information for full synthetic details. aIsolated yield on 5.0 mmol scale, with 8 mol % of 4·[O]. bReaction conducted with Ph2SiH2 (4.4 equiv) in MeCN. cReaction conducted at 60 °C for 20 h. dReaction conducted on 0.2 mmol scale using 15 mol % of [4·Br]Br in MeCN at 50 °C for 20–40 h. eYield determined by 1H NMR with internal standard. fReaction conducted with DEMBM. DCE = 1,2-dichloroethane; Bn = benzyl; Ts = tosyl; iBu = isobutyl.
When N-alkyl amine substrates were initially employed under standard conditions, an undesired byproduct arising from N-alkylation by DEBM was identified by gas chromatography–mass spectrometry (GCMS). However, replacing DEBM with its methyl-substituted analogue DEMBM abated this deleterious pathway, restoring the high degree of redox compatibility necessary for the catalytic system. Consequently, o-pyrrolobenzylamine could be efficiently coupled with carboxylic acids via iterative dehydration to provide the corresponding pyrrolobenzodiazepines, a prevalent bioactive scaffold,24 in good yields (3s–3u, 66–86% yields).25 Heterocycles 3t and 3u, compounds investigated by Janssen for their antifungal activity,26 could be prepared in a single synthetic operation in 86% and 73% yields, respectively. Further, tryptamines and phenethylamines could be transformed into dihydro-β-carboline and dihydroisoquinoline products in good yields (3v–3x, 70–77% yields).27 Notably, both of these scaffolds are found in bioactive pharmaceutical agents and natural products,28 as demonstrated by the assembly in a single step of dihydroisoquinoline natural product 3,4-dihydropapaverine from commercial starting materials with good efficiency (3x, 70% yield).
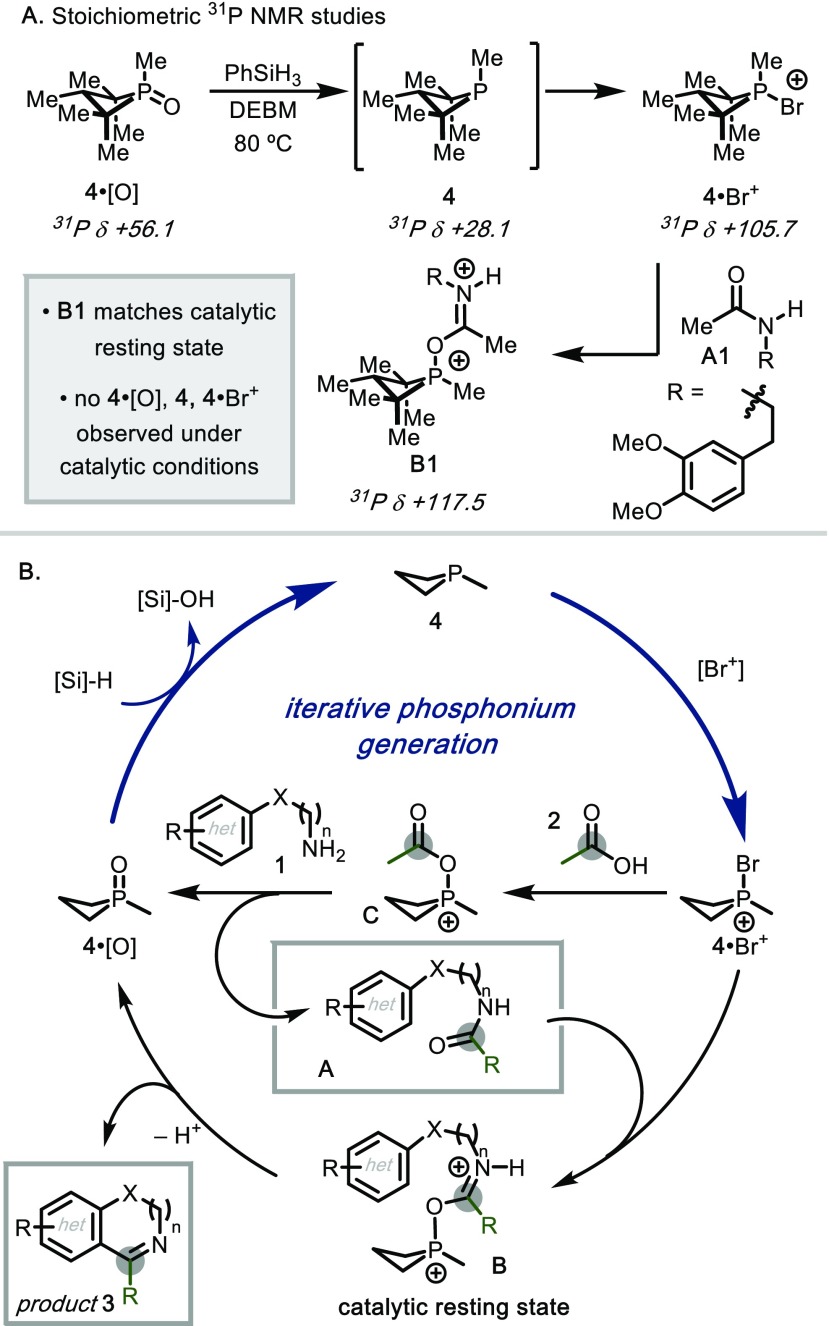
Concerning the catalytic mechanism, in situ 1H NMR spectroscopy revealed a rapid initial conversion of reactants 1a and 2a into amide intermediate 3a′, followed by comparatively slow formation of heterocycle 3a (see Supporting Information, Section VI), establishing a stepwise reaction sequence for the autotandem catalytic process in which C–C bond-forming cyclodehydration is kinetically limiting. Despite the observation that 4, 4·[O], and 4·Br+ are each competent precatalysts (vide supra), in situ 31P NMR and direct analysis in real time (DART) mass spectrometry (MS) analyses show that none of these compounds represent the catalytic resting state. Rather, experiments are most consistent with resting state B1, which is an adduct of the phosphacyclic catalyst and amide A1 (Figure 3A). Indeed, independent reaction of [4·Br]Br with A1 gives rise to spectroscopic signals indistinguishable from those observed under the catalytic steady state, and this species was shown to lead to C–C bond-forming cyclodehydration.29 Furthermore, spectroscopically indistinguishable species are observed by 31P NMR spectroscopy when either N-methylacetamide or N,N-dimethylacetamide are introduced in lieu of reactive amides to a mixture containing catalytic components (i.e., 4·[O], PhSiH3, DEBM).
Figure 3.
(A) 31P NMR studies, resonance of major diastereomer. (B) Proposed mechanism of autotandem catalytic dehydrative annulation of amines and carboxylic acids. Methyl groups excluded from 4 for clarity.
Figure 3B depicts a plausible autotandem catalytic reaction mechanism consistent with the foregoing experimental data. From phosphine oxide 4·[O] as precatalyst, entry to the C–N bond-forming cycle (Figure 3B) is initiated by kinetically facile phenylsilane-mediated reduction to phosphine 4, followed by rapid halophilic reaction30 with DEBM leading to bromophosphonium ion 4·Br+. Bromophosphonium cation 4·Br+ effects intermolecular amidation between acid 1 and amine 2, presumably via intermediate C in analogy to established precedent for amine N-acylation by activated acyloxyphosphoniums, thereby returning phosphine oxide 4·[O]. In C–C bond-forming second phase, phosphonium ion 4·Br+ is again generated by a reduction–oxidation sequence with PhSiH3 and DEBM, respectively. Exchange of bromide for the amide substrate A then leads to activated species B, which is assigned as the catalytic resting state. Cyclization ensues to provide the product 3, liberating phosphine oxide 4·[O] and closing the catalytic cycle.31 The two noteworthy conclusions emerging from this mechanistic picture are (1) turnover of phosphine oxide 4·[O] to phosphine 4 is not kinetically limiting and (2) the concentration of reducing phosphine 4 remains negligibly low during catalysis as a function of the efficient reaction with the oxidative halenium shunt.
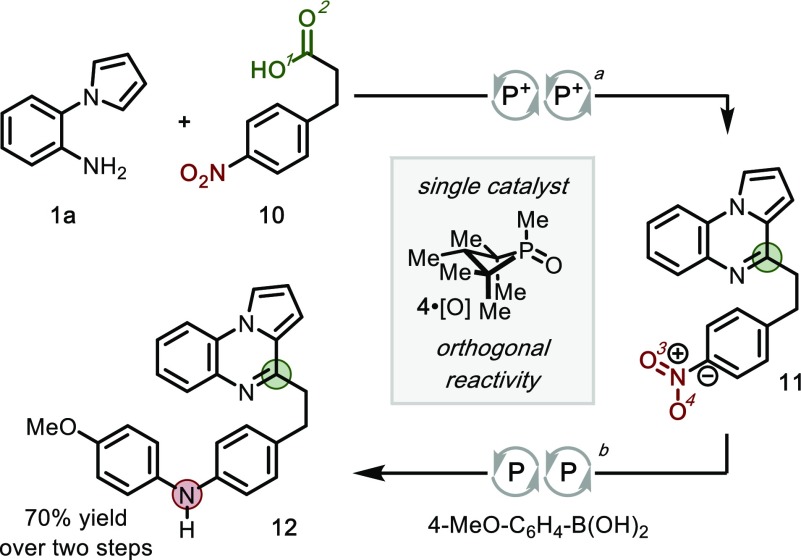
The net redox neutral character of the recursive dehydration (and the absence of appreciable concentrations of phosphine 4) enables chemoselective annulation in preference to established PIII/PV-catalyzed reductive transformations. This orthogonality is illustrated in the context of p-nitrohydrocinnamic acid (10), possessing both carboxylic acid and nitroarene moieties (Figure 4). Under the recursive dehydration conditions described herein, catalyst 4·[O] drives the selective annulation of carboxylic acid 10 to yield pyrroloquinoxaline 11 in 97% yield. With the same catalyst (4·[O]) but omission of the DEBM shunt, the nitro group is then reductively addressed to effect intermolecular C–N cross coupling9c and yield fully deoxygenated species 12 in 72% yield. This sequence, in which a single organophosphorus catalyst executes four distinct oxygen excisions, demonstrates the complementarity of the reductive and redox-neutral PIII/PV-catalytic manifolds owing to the disparate reactivity of the phosphetane in its different oxidation states as a function of the addition or exclusion of an exogenous oxidant.
Figure 4.
Selective functionalization of carboxylic acid- and nitro-containing substrate via sequential redox-neutral recursive dehydration, then reductive recursive deoxygenation, using a single catalyst 4·[O]. Reaction conditions: (a) 1a (1.0 equiv), 10 (1.05 equiv), DEBM (2.4 equiv), PhSiH3 (2.2 equiv), 4·[O] (15 mol %), DCE, 80 °C; (b) 11 (1.0 equiv), 4-MeO-C6H4-B(OH)2 (1.1 equiv), PhSiH3 (2.0 equiv), 4·[O] (15 mol %), m-xylene, 120 °C.
In conclusion, we have demonstrated that a small-ring phosphetane catalyst can induce iterative dehydrative C–N and C–C bond-forming reactions, enabling direct azaheterocycle synthesis from carboxylic acids and amines via recursive dehydration. Through the synergistic use of mild hydrosilane reductant and bromenium oxidant, the elements of water can be catalytically removed in the form of an O-atom and two protons with complete redox compatibility. We anticipate that this phosphacatalytic dehydration manifold will prove generally enabling for the redox-neutral functionalization of oxygenated organic functionalities to accomplish C–C and C–heteroatom bond-forming events via condensation, especially in a recursive fashion.
Acknowledgments
Financial support was provided by NIH NIGMS (GM114547). M.L. thanks the Belgian American Educational Foundation for a postdoctoral fellowship. J.M.L. thanks the Camille and Henry Dreyfus Foundation for a postdoctoral fellowship in Environmental Chemistry. S.-H.K.-L. thanks Prof. P. Mauleón, Univ. Autónoma de Madrid, for a mobility grant and Ministerio de Educación, Cultura y Deporte (MECD) for a FPU predoctoral fellowship. The authors acknowledge the Buchwald laboratory (MIT) for access to HPLC equipment, and J. C. Gilhula and H. W. Moon for assistance in acquiring HRMS data.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b06277.
Additional optimization results, mechanistic studies, and synthetic procedures. 1H, 13C, and 31P NMR spectra (PDF)
Author Contributions
§ Authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a O’Brien C. J.; Tellez J. L.; Nixon Z. S.; Kang L. J.; Carter A. L.; Kunkel S. R.; Przeworski K. C.; Chass G. A. Recycling the Waste: The Development of a Catalytic Wittig Reaction. Angew. Chem., Int. Ed. 2009, 48, 6836–6839. 10.1002/anie.200902525. [DOI] [PubMed] [Google Scholar]; b O’Brien C. J.Catalytic Wittig and Mitsunobu Reactions. U.S. Patent 8,901,365, Dec 2, 2014.
- a van Kalkeren H. A.; van Delft F. L.; Rutjes F. P. J. T. Organophosphorus Catalysis to Bypass Phosphine Oxide Waste. ChemSusChem 2013, 6, 1615–1624. 10.1002/cssc.201300368. [DOI] [PubMed] [Google Scholar]; b Guo H.; Fan Y. C.; Sun Z.; Wu Y.; Kwon O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews discussing PV=O catalysis, see:; a Marsden S. P.Catalytic Variants of Phosphine Oxide-Mediated Organic Transformations. In Sustainable Catalysis ;Dunn P. J., Hii K. K., Krische M. J., Williams M. T., Eds.; John Wiley & Sons, Inc.: New York, 2013; pp 339–361. [Google Scholar]; b Denmark S. E.; Stavenger R. A. Asymmetric Catalysis of Aldol Reactions with Chiral Lewis Bases. Acc. Chem. Res. 2000, 33, 432–440. 10.1021/ar960027g. [DOI] [PubMed] [Google Scholar]; c Denmark S. E.; Beutner G. L. Lewis Base Catalysis in Organic Synthesis. Angew. Chem., Int. Ed. 2008, 47, 1560–1638. 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]; d Benaglia M.; Rossi S. Chiral Phosphine Oxides in Present-Day Organocatalysis. Org. Biomol. Chem. 2010, 8, 3824–3830. 10.1039/c004681g. [DOI] [PubMed] [Google Scholar]
- Marsi K. L. Phenylsilane Reduction of Phosphine Oxides with Complete Stereospecificity. J. Org. Chem. 1974, 39, 265–267. 10.1021/jo00916a041. [DOI] [Google Scholar]
- a van Kalkeren H. A.; Leenders S. H. A. M.; Hommersom C. R. A.; Rutjes F. P. J. T.; van Delft F. L. In Situ Phosphine Oxide Reduction: A Catalytic Appel Reaction. Chem. - Eur. J. 2011, 17, 11290–11295. 10.1002/chem.201101563. [DOI] [PubMed] [Google Scholar]; b van Kalkeren H. A.; van Delft F. L.; Rutjes F. P. J. T. Catalytic Appel Reactions. Pure Appl. Chem. 2012, 85, 817–828. 10.1351/PAC-CON-12-06-13. [DOI] [Google Scholar]
- Zhao W.; Yan P. K.; Radosevich A. T. A Phosphetane Catalyzes Deoxygenative Condensation of α-Keto Esters and Carboxylic Acids via PIII/PV=O Redox Cycling. J. Am. Chem. Soc. 2015, 137, 616–619. 10.1021/ja511889y. [DOI] [PubMed] [Google Scholar]
- For phosphacatalytic Staudinger and related reactions:; a van Kalkeren H. A.; Bruins J. J.; Rutjes F. P. J. T.; van Delft F. L. Organophosphorus-Catalysed Staudinger Reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. 10.1002/adsc.201100967. [DOI] [Google Scholar]; b van Kalkeren H. A.; te Grotenhuis C.; Haasjes F. S.; Hommersom C. R. A.; Rutjes F. P. J. T.; van Delft F. L. Catalytic Staudinger/Aza-Wittig Sequence by In Situ Phosphane Oxide Reduction. Eur. J. Org. Chem. 2013, 2013, 7059–7066. 10.1002/ejoc.201300585. [DOI] [Google Scholar]; c Cai L.; Zhang K.; Chen S.; Lepage R. J.; Houk K. N.; Krenske E. H.; Kwon O. Catalytic Asymmetric Staudinger-Aza-Wittig Reaction for the Synthesis of Heterocyclic Amines. J. Am. Chem. Soc. 2019, 141, 9537–9542. 10.1021/jacs.9b04803. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kosal A. D.; Wilson E. E.; Ashfeld B. L. Phosphine-Based Redox Catalysis in the Direct Traceless Staudinger Ligation of Carboxylic Acids and Azides. Angew. Chem., Int. Ed. 2012, 51, 12036–12040. 10.1002/anie.201206533. [DOI] [PubMed] [Google Scholar]
- For phosphacatalytic Wittig and related reactions:; a O’Brien C. J.; Lavigne F.; Coyle E. E.; Holohan A. J.; Doonan B. J. Breaking the Ring Through a Room Temperature Catalytic Wittig Reaction. Chem. - Eur. J. 2013, 19, 5854–5858. 10.1002/chem.201300546. [DOI] [PubMed] [Google Scholar]; b O’Brien C. J.; Nixon Z. S.; Holohan A. J.; Kunkel S. R.; Tellez J. L.; Doonan B. J.; Coyle E. E.; Lavigne F.; Kang L. J.; Przeworski K. C. Part I: The Development of the Catalytic Wittig Reaction. Chem. - Eur. J. 2013, 19, 15281–15289. 10.1002/chem.201301444. [DOI] [PubMed] [Google Scholar]; c Coyle E. E.; Doonan B. J.; Holohan A. J.; Walsh K. A.; Lavigne F.; Krenske E. H.; O’Brien C. J. Catalytic Wittig Reactions of Semi- and Nonstabilized Ylides Enabled by Ylide Tuning. Angew. Chem., Int. Ed. 2014, 53, 12907–12911. 10.1002/anie.201406103. [DOI] [PubMed] [Google Scholar]; d Lee C.; Chang T.; Yu J.; Reddy G. M.; Hsiao M.; Lin W. Synthesis of Functionalized Furans via Chemoselective Reduction/Wittig Reaction Using Catalytic Triethylamine and Phosphine. Org. Lett. 2016, 18, 3758–3761. 10.1021/acs.orglett.6b01781. [DOI] [PubMed] [Google Scholar]; e Saleh N.; Voituriez A. Synthesis of 9H-Pyrrolo[1,2-a]indole and 3H-Pyrrolizine Derivatives via a Phosphine-Catalyzed Umpolung Addition/Intramolecular Wittig Reaction. J. Org. Chem. 2016, 81, 4371–4377. 10.1021/acs.joc.6b00473. [DOI] [PubMed] [Google Scholar]; f Saleh N.; Blanchard F.; Voituriez A. Synthesis of Nitrogen Containing Heterocycles and Cyclopentenone Derivatives via Phosphine Catalyzed Michael Addition/Intramolecular Wittig Reaction. Adv. Synth. Catal. 2017, 359, 2304–2315. 10.1002/adsc.201700313. [DOI] [Google Scholar]; g Zhang K.; Cai L.; Yang Z.; Houk K. N.; Kwon O. Bridged [2.2.1] Bicyclic Phosphine Oxide Facilitates Catalytic γ-Umpolung Addition-Wittig Olefination. Chem. Sci. 2018, 9, 1867–1872. 10.1039/C7SC04381C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nykaza T. V.; Harrison T. S.; Ghosh A.; Putnik R. A.; Radosevich A. T. A Biphilic Phosphetane Catalyzes N–N Bond-Forming Cadogan Heterocyclization via PIII/PV=O Redox Cycling. J. Am. Chem. Soc. 2017, 139, 6839–6842. 10.1021/jacs.7b03260. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nykaza T. V.; Ramirez A.; Harrison T. S.; Luzung M. R.; Radosevich A. T. Biphilic Organophosphorus-Catalyzed Intramolecular Csp2–H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc. 2018, 140, 3103–3113. 10.1021/jacs.7b13803. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nykaza T. V.; Cooper J. C.; Li G.; Mahieu N.; Ramirez A.; Luzung M. R.; Radosevich A. T. Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc. 2018, 140, 15200–15205. 10.1021/jacs.8b10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.; Lecomte M.; Kim-Lee S.-H.; Radosevich A. T. Organophosphorus-Catalyzed Deoxygenation of Sulfonyl Chlorides: Electrophilic (Fluoroalkyl)sulfenylation by PIII/PV=P Redox Cycling. Angew. Chem., Int. Ed. 2019, 58, 2864–2869. 10.1002/anie.201813919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg D. E.; dos Santos E. N. Tandem Catalysis: A Taxonomy and Illustrative Review. Coord. Chem. Rev. 2004, 248, 2365–2379. 10.1016/j.ccr.2004.05.012. [DOI] [Google Scholar]
- a Mukaiyama T. Oxidation-Reduction Condensation. Angew. Chem., Int. Ed. Engl. 1976, 15, 94–103. 10.1002/anie.197600941. [DOI] [Google Scholar]; b Mukaiyama T. Explorations into New Reaction Chemistry. Angew. Chem., Int. Ed. 2004, 43, 5590–5614. 10.1002/anie.200300641. [DOI] [PubMed] [Google Scholar]
- a Buonomo J. A.; Aldrich C. C. Mitsunobu Reactions Catalytic in Phosphine and a Fully Catalytic System. Angew. Chem., Int. Ed. 2015, 54, 13041–13044. 10.1002/anie.201506263. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hirose D.; Gazvoda M.; Košmrlj J.; Taniguchi T. The “Fully Catalytic System” in Mitsunobu Reaction Has Not Been Realized Yet. Org. Lett. 2016, 18, 4036–4039. 10.1021/acs.orglett.6b01894. [DOI] [PubMed] [Google Scholar]; c Beddoe R. H.; Sneddon H. F.; Denton R. M. The Catalytic Mitsunobu Reaction: A Critical Analysis of the Current State-of-the-Art. Org. Biomol. Chem. 2018, 16, 7774–7781. 10.1039/C8OB01929K. [DOI] [PubMed] [Google Scholar]
- Classical Appel conditions have been adapted for N-acylation reactions:; a Appel R. Tertiary Phosphane/Tetrachloromethane, a Versatile Reagent for Chlorination, Dehydration, and P–N Linkage. Angew. Chem., Int. Ed. Engl. 1975, 14, 801–811. 10.1002/anie.197508011. [DOI] [Google Scholar]; b Barstow L. E.; Hruby V. J. A Simple Method for the Synthesis of Amides. J. Org. Chem. 1971, 36, 1305–1306. 10.1021/jo00808a033. [DOI] [Google Scholar]; c Luo Q.-L.; Lv L.; Li Y.; Tan J.-P.; Nan W.; Hui Q. An Efficient Protocol for the Amidation of Carboxylic Acids Promoted by Trimethyl Phosphite and Iodine. Eur. J. Org. Chem. 2011, 2011, 6916–6922. 10.1002/ejoc.201101030. [DOI] [Google Scholar]
- For halophosphonium cations as Lewis acids, see:; a Caputo C. B.; Hounjet L. J.; Dobrovetsky R.; Stephan D. W. Lewis Acidity of Organofluorophosphonium Salts: Hydrodefluorination by a Saturated Acceptor. Science 2013, 341, 1374–1377. 10.1126/science.1241764. [DOI] [PubMed] [Google Scholar]; b Bayne J. M.; Stephan D. W. Phosphorus Lewis Acids: Emerging Reactivity and Applications in Catalysis. Chem. Soc. Rev. 2016, 45, 765–774. [DOI] [PubMed] [Google Scholar]
- Lenstra D. C.; Rutjes F. P. J. T.; Mecinović J. Triphenylphosphine-Catalysed Amide Bond Formation Between Carboxylic Acids and Amines. Chem. Commun. 2014, 50, 5763–5766. 10.1039/c4cc01861c. [DOI] [PubMed] [Google Scholar]
- PV-catalyzed deoxychlorination and amidation reactions using phosphine oxide and oxalyl chloride have been developed:; a Denton R. M.; An J.; Adeniran B. Phosphine Oxide-Catalysed Chlorination Reactions of Alcohols Under Appel Conditions. Chem. Commun. 2010, 46, 3025–3027. 10.1039/c002825h. [DOI] [PubMed] [Google Scholar]; b Denton R. M.; Tang X.; Przeslak A. Catalysis of Phosphorus(V)-Mediated Transformations: Dichlorination Reactions of Epoxides Under Appel Conditions. Org. Lett. 2010, 12, 4678–4681. 10.1021/ol102010h. [DOI] [PubMed] [Google Scholar]; c Denton R. M.; An J.; Adeniran B.; Blake A. J.; Lewis W.; Poulton A. M. Catalytic Phosphorus(V)-Mediated Nucleophilic Substitution Reactions: Development of a Catalytic Appel Reaction. J. Org. Chem. 2011, 76, 6749–6767. 10.1021/jo201085r. [DOI] [PubMed] [Google Scholar]; d An J.; Tang X.; Moore J.; Lewis W.; Denton R. M. Phosphorus(V)-Catalyzed Deoxydichlorination Reactions of Aldehydes. Tetrahedron 2013, 69, 8769–8776. 10.1016/j.tet.2013.07.100. [DOI] [Google Scholar]; e Yu T.-Y.; Wang Y.; Xu P.-F. An Unusual Triphenylphosphine Oxide Catalyzed Stereoselective 1,3-Dichlorination of Unsaturated Ketoesters. Chem. - Eur. J. 2014, 20, 98–101. 10.1002/chem.201303688. [DOI] [PubMed] [Google Scholar]; f Tang X.; An J.; Denton R. M. A Procedure for Appel Halogenations and Dehydrations Using a Polystyrene Supported Phosphine Oxide. Tetrahedron Lett. 2014, 55, 799–802. 10.1016/j.tetlet.2013.11.098. [DOI] [Google Scholar]; g Jiang L.; Yu J.; Niu F.; Zhang D.; Sun X. A High-Efficient Method for the Amidation of Carboxylic Acids Promoted by Triphenylphosphine Oxide and Oxalyl Chloride. Heteroat. Chem. 2017, 28, e21364 10.1002/hc.21364. [DOI] [Google Scholar]
- Hendrickson J. B.; Hussoin M. S. Seeking the Ideal Dehydrating Reagent. J. Org. Chem. 1987, 52, 4137–4139. 10.1021/jo00227a041. [DOI] [Google Scholar]
- Phosphonium salts are widely used as peptide-coupling reagents:; a El-Faham A.; Albericio F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]; b Berchel M.; Jaffrès P.-A.. Recent Developments in Phosphonium Chemistry. In Organophosphorus Chemistry: From Molecules to Applications ;Iaroshenko V., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp 78–84. [Google Scholar]
- Strem item No. 15-8150.
- Consistent with the notion of sequential C–N then C–C bond forming by serial dehydration events, the optimized protocol using DEBM can be applied to access a wide variety of azaheterocycles via catalytic Bischler-Napieralski-type cyclodehydration of preformed amides (see Supporting Information, Section V, Table S4, 18 examples).
- Silanes have been shown to promote amidation, including in phosphine-catalyzed Staudinger amidation. However, low background conversion to amide was observed with PhSiH3 in the absence of either 4·[O] or DEBM (see Supporting Information, Section II, Table S1). See:; a Ruan Z.; Lawrence R. M.; Cooper C. B. Phenylsilane as an Active Amidation Reagent for the Preparation of Carboxamides and Peptides. Tetrahedron Lett. 2006, 47, 7649–7651. 10.1016/j.tetlet.2006.08.024. [DOI] [Google Scholar]; b Sayes M.; Charette A. B. Diphenylsilane as a Coupling Reagent for Amide Bond Formation. Green Chem. 2017, 19, 5060–5064. 10.1039/C7GC02643A. [DOI] [Google Scholar]; c Andrews K. G.; Denton R. M. A More Critical Role for Silicon in the Catalytic Staudinger Amidation: Silanes as Non-Innocent Reductants. Chem. Commun. 2017, 53, 7982–7985. 10.1039/C7CC03076B. [DOI] [PubMed] [Google Scholar]
- For the synthesis of halophosphoniums from phosphine oxides, see:; a Nikitin K.; Müller-Bunz H.; Gilheany D. Direct Evidence of a Multicentre Halogen Bond: Unexpected Contraction of the P–XXX–P Fragment in Triphenylphosphine Dihalides. Chem. Commun. 2013, 49, 1434–1436. 10.1039/c2cc38363b. [DOI] [PubMed] [Google Scholar]; b Jennings E. V.; Nikitin K.; Ortin Y.; Gilheany D. G. Degenerate Nucleophilic Substitution in Phosphonium Salts. J. Am. Chem. Soc. 2014, 136, 16217–16226. 10.1021/ja507433g. [DOI] [PubMed] [Google Scholar]; c Nikitin K.; Jennings E. V.; Al Sulaimi S.; Ortin Y.; Gilheany D. G. Dynamic Cross-Exchange in Halophosphonium Species: Direct Observation of Stereochemical Inversion in the Course of an SN2 Process. Angew. Chem., Int. Ed. 2018, 57, 1480–1484. 10.1002/anie.201708649. [DOI] [PubMed] [Google Scholar]
- a Gerratana B. Biosynthesis, Synthesis, and Biological Activities of Pyrrolobenzodiazepines. Med. Res. Rev. 2012, 32, 254–293. 10.1002/med.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hartley J. A. The Development of Pyrrolobenzodiazapines as Antitumor Agents. Expert Opin. Invest. Drugs 2011, 20, 733–744. 10.1517/13543784.2011.573477. [DOI] [PubMed] [Google Scholar]; c Dornisch E.; Pletz J.; Glabonjat R. A.; Martin F.; Lembacher-Fadum C.; Neger M.; Högenauer C.; Francesconi K.; Kroutil W.; Zangger K.; Breinbauer R.; Zechner E. L. Biosynthesis of the Enterotoxic Pyrrolobenzodiazepine Natural Product Tilivalline. Angew. Chem., Int. Ed. 2017, 56, 14753–14757. 10.1002/anie.201707737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For comparison, 3s was formed in 31% yield using DEBM (yield by 1H NMR analysis with the aid of an internal standard).
- a Meerpoel L.; Van Gestel J.; Van Gerven F.; Woestenborghs F.; Marichal P.; Sipido V.; Terence G.; Nash R.; Corens D.; Richards R. D. Pyrrolo[1,2-a][1,4]benzodiazepine: A Novel Class of Non-Azole Anti-Dermatophyte Anti-Fungal Agents. Bioorg. Med. Chem. Lett. 2005, 15, 3453–3458. 10.1016/j.bmcl.2005.05.007. [DOI] [PubMed] [Google Scholar]; b Paulussen C.; de Wit K.; Boulet G.; Cos P.; Meerpoel L.; Maes L. Pyrrolo[1,2-α][1,4]benzodiazepines Show Potent In Vitro Antifungal Activity and Significant In Vivo Efficacy in a Microsporum canis Dermatitis Model in Guinea Pigs. J. Antimicrob. Chemother. 2014, 69, 1608–1610. 10.1093/jac/dku034. [DOI] [PubMed] [Google Scholar]
- Compound 3w is formed racemically under both standard and modified conditions (as used for 3p-3s). 3,4-Dihydroisoquinolines bearing α-stereogenic centers are known to undergo facile thermal racemization even at room temperature; see:Movassaghi M.; Hill M. D. A Versatile Cyclodehydration Reaction for the Synthesis of Isoquinoline and β-Carboline Derivatives. Org. Lett. 2008, 10, 3485–3488. 10.1021/ol801264u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a McKenzie E.; Nettleship L.; Slaytor M. New Natural Products from Peganum harmala. Phytochemistry 1975, 14, 273–275. 10.1016/0031-9422(75)85054-0. [DOI] [Google Scholar]; b Cao R.; Peng W.; Wang Z.; Xu A. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]; c Rommelspacher H.; Susilo R.. Tetrahydroisoquinolines and β-Carbolines: Putative Natural Substances in Plants and Mammals. In Progress in Drug Research ;Jucker E., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1985; Vol. 29, pp 415–459. [DOI] [PubMed] [Google Scholar]
- Whaley W. M.; Govindachari T. R. The Preparation of 3,4-Dihydroisoquinolines and Related Compounds by the Bischler-Napieralski Reaction. Org. React. 1951, 6, 74–144. [Google Scholar]
- a Hoffmann H.; Diehr H. J. Phosphonium Salt Formation of the Second Kind. Angew. Chem., Int. Ed. Engl. 1964, 3, 737–746. 10.1002/anie.196407371. [DOI] [Google Scholar]; b Zefirov N. S.; Makhon’kov D. I. X-philic Reactions. Chem. Rev. 1982, 82, 615–624. 10.1021/cr00052a004. [DOI] [Google Scholar]
- The classical Bischler-Napieralski reaction is understood to proceed via elimination of oxyphosphonium to generate a nitrilium ion, as evidenced by the formation of side-product alkene deriving from retro-Ritter reaction. For no substrates described in this manuscript was alkene formation observed, but the intermediacy of a nitrilium ion cannot be ruled out in this chemistry. See:; a Fodor G.; Nagubandi S. Correlation of the von Braun, Ritter, Bischler-Napieralski, Beckmann and Schmidt Reactions via Nitrilium Salt Intermediates. Tetrahedron 1980, 36, 1279–1300. 10.1016/0040-4020(80)85039-3. [DOI] [Google Scholar]; b Nagubandi S.; Fodor G. The Mechanism of the Bischler-Napieralski Reaction. J. Heterocycl. Chem. 1980, 17, 1457–1463. 10.1002/jhet.5570170720. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.