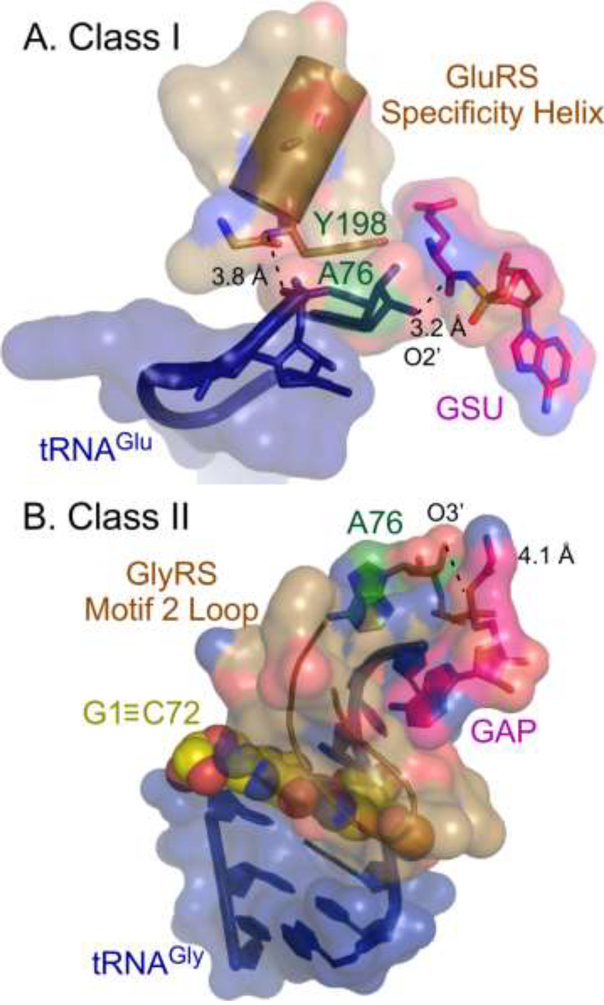
Figure 3.
Acylation complexes of Class I and II cognate pairs. Urzymes of both Class I and II aaRS afford secondary structural features that recognize cognate tRNAs. In both complexes the terminal A76 is green, the activated amino acid analog is magenta, and the tRNA is dark blue. A. Class I GluRS complex illustrates the role played by the amino terminus of the specificity helix (sand) in recognition of the hairpin characteristic of Class I-cognate tRNAs. The recognition complex is reinforced by a conserved aromatic residue (i.e. Y198) near the N-terminus of that helix. B. Class II GlyRS complex illustrates the role of the Motif 2 loop in stabilizing the helical extension of the CCA 3’ terminus by lying atop the topmost base pair (G1≡C72; yellow) of the acceptor stem. Distances are given between the nucleophilic ribose OH group and the aminoacyl group of the activated amino acid analog and between an amino group at the N-terminus of the specificity-determining helix in GluRS and the A76 phosphate group.