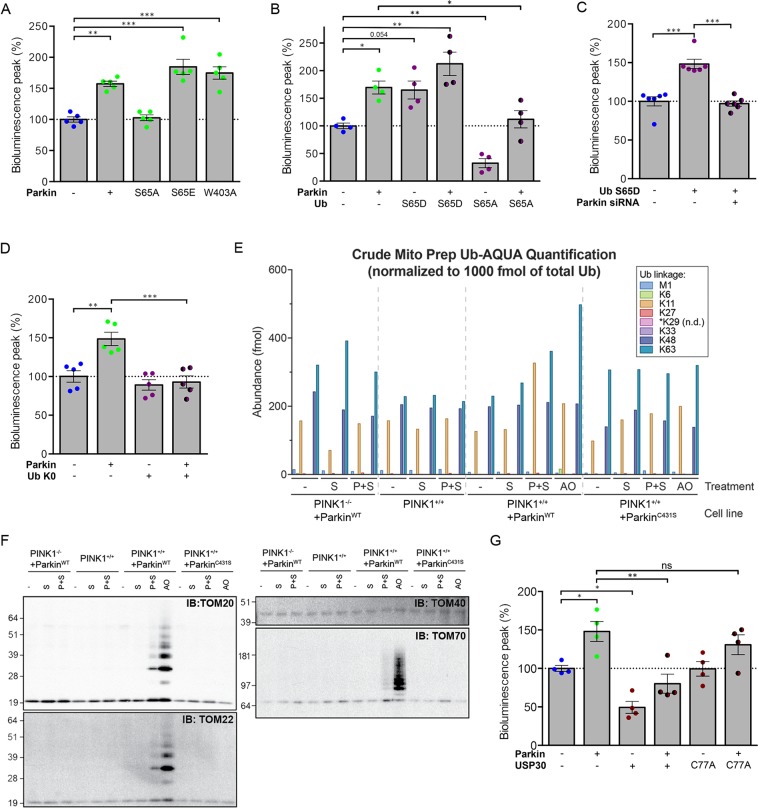
Figure 4.
Parkin modulates mitochondrial import through its E3 ubiquitin-protein ligase activity induced by PINK1. Effect of relief of Parkin autoinhibition by (A) the activating W403A substitution, or by mimicking the PINK1-mediated phosphorylation of Parkin UBL (S65E) or (B) Ub (S65D), showing exacerbation of the Parkin-dependent enhancement of the bioluminescent signal of Probe 2 in HEK293T cells. By contrast, non-phosphorylatable S65A Parkin or Ub variants abolish the effect of Parkin. (C) In these cells, Ub S65D mimics the effect of Parkin overproduction in a manner dependent on endogenous Parkin (Parkin siRNA). (D) Effect of the lysine-less Ub K0 variant on the intensity of the bioluminescent signal of Probe 2 in HEK293T, indicating that the effect of Parkin is mediated by polyubiquitylation. (E) Quantification by UB-AQUA proteomics of individual Ub chain linkage types associated with mitochondria in HeLa Flp-In T-REx ParkinWT or ParkinC431S cells depleted or not of PINK1 and expressing Probe 2 in the presence of Shield1 (P + S), or treated with S or AO. (F) Immunoblot analysis of ubiquitylated proteins pulled down with TUBEs from mitochondrion-enriched fractions analyzed in (E) shows ubiquitylation of the TOM subunits TOM20, TOM22 and TOM70 in cells expressing Probe 2 (P + S). (G) Analysis of the impact of the mitochondrial ubiquitin-specific protease USP30 or its inactive C77A variant on the signal of Probe in the presence or absence of overexpressed Parkin. n = 4 (A,B,G), 5 (D) or 6 (C) independent experiments. Results are expressed as means ± SEM. Data were analyzed by one-way (A) or two-way ANOVA (B,C,G) with Dunnett’s or Holm-Sidak’s posthoc tests. *p < 0.05, **p < 0.01, ***p < 0.001. ns: non-significant.