Abstract
Differentiation of Guillain–Barré syndrome (GBS) and acute-onset chronic inflammatory demyelinating polyradiculoneuritis (CIDP) might be intricate in early stages. We compared electrodiagnostics (EDx) and nerve ultrasound (NUS) as tools for early distinction and follow-up. NUS and EDx have been performed at first visitation and after 6 months. The nerve conduction study score (NCSS), the ultrasound pattern sum score (UPSS), and clinical scores were used for comparison. Compared with the 33 GBS patients, the 34 CIDP patients (50% with symptoms < 4 weeks) revealed significant nerve enlargement in ultrasound (p < 0.001) except for the roots and vagus, which exhibited increased values in both groups. EDx has no significant differences between both groups except for the A-wave frequency and the sural sparing pattern, which is more frequent in GBS (Fisher’s exact p < 0.05). In the latter, particularly, pure sensory nerves were not enlarged in contrast to CIDP, in which those were mostly enlarged (p < 0.001). This ultrasonic sensory sparing pattern (uSSP) in combination with enlarged roots/vagus is the hallmark finding in GBS with sensitivity, specificity, and positive predictive value > 85%, whereas in CIDP, enlarged sensory und multifocally enlarged sensorimotor nerves are key differentiation features to GBS. Increased echointensity of the nerves further arises only in CIDP. After 6 months, in CIDP, the significant nerve enlargement persisted, whereas in GBS, all segments almost normalized. Clinical, ultrasonic, and NCS scores correlated significantly over the time. Enlarged roots/vagus in combination with uSSP might facilitate differentiation of GBS and CIDP in the early stage, and ultrasonic 6-month normalization underlines the diagnosis of GBS in cases of uncertainty. Trial Registration: DRKS-ID 00005253
Electronic supplementary material
The online version of this article (10.1007/s13311-019-00716-5) contains supplementary material, which is available to authorized users.
Key Words: CIDP, GBS, high-resolution ultrasound, UPSS, immune-mediated neuropathies
Introduction
Acute immune-mediated neuropathies, summarized as Guillain–Barré syndrome (GBS), most often differ from the chronic inflammatory demyelinating polyradiculoneuritis (CIDP) by its rapid onset in between two to four weeks, often preceded by infections, affection of cranial nerves, and autonomic dysregulation, whereas chronic neuropathies mostly progress over weeks to months continuously or stepwise without significant autonomic dysregulation or cranial nerve palsies [1–3].
However, onset and course of inflammatory neuropathies sometimes might be similar in the early phase of the disease, particularly between weeks 4 and 8, with rapid onset of distal sensory disturbances, ataxia, and progressive flaccid paraparesis or tetraparesis [4]. Early differentiation by clinical and technical diagnostics, such as laboratory markers (cerebrospinal fluid (CSF), antibodies, etc.), clinical neurophysiological methods, and clinical scores as well, might be ambiguous in many cases; even the electrophysiological sural sparing pattern (eSSP) seems to emerge in both entities. However, therapy and prognosis differ significantly between these entities [1–3].
Recent studies suggest that a differentiation might be possible by the application of ultrasound pattern sum score (UPSS) or the Bochum ultrasound score with more prominent nerve enlargement in CIDP than in GBS variants [5–7], but ultrasound data for acute-onset CIDP are sparse. It has been shown that in both entities, nerve enlargement might regress under therapy [8–10], but a systematic comparison between acute and chronic immune-mediated neuropathies is still missing. Therefore, patients with GBS and acute/subacute CIDP variants were comparatively analyzed over a 6-month period using ultrasound and clinical and electrophysiological scores.
Methods
Patients newly diagnosed with acute or chronic immune-mediated neuropathy were included. All acute variants, i.e., acute inflammatory demyelinating polyradiculoneuritis (AIDP), acute (sensori)motor axonal neuritis (AM(S)AN), or Miller–Fisher syndrome (MFS), were summarized as GBS, and all chronic variants, including Lewis–Sumner syndrome, were summarized as CIDP. The study was registered in the German clinical trial registry (DRKS-ID 00005253) and approved by the local ethics committee (Tübingen 702/2015BO2). Written informed consent to study participation was obtained from all patients. The diagnosis of immune-mediated neuropathy was done by EFNS guidelines as well as according to Asbury et al. [3, 11] as recommended. However, the diagnosis of chronic versus acute neuropathy was often done retrospectively by its clinical course and development during the observational period.
Time interval (in weeks) from first symptom onset to the first presentation at our departments was evaluated. Patients with symptom onset more than 9 months before first visit were not included in our study. Detailed clinical examination was performed in all patients. Motor impairment was scored using the medical research council sum score (MRCSS, range 0 with complete paralysis of all 12 analyzed muscles to 60 points with preserved muscle strength in all muscles), and overall disturbances were rated by the INCAT overall disability score (0 points is no disturbances, 10 points is severe impairment with almost all functions of daily lifetime restricted, [12, 13]). The change of both scores was evaluated as Δ (= difference of follow-up result and onset result).
All patients received blood serum samples looking for acute inflammation markers, i.e., human immodeficency virus (HIV), borreliosis, treponema, paraproteinemia, vasculitis, or vitamin deficiency. Cerebrospinal fluid was analyzed for cell count, protein, glucosis, and albumin ratio and a protein elevation > 50 mg/dl or a cell count > 5 cells/μl were rated as pathologic. In most patients (n = 23 GBS and n = 20 CIDP patients), ganglioside antibodies were analyzed. Magnetic resonance imaging of the lumbar roots with gadolinium application was evaluated if available (n = 19 GBS and n = 21 CIDP). Gadolinium (Gd) enhancement or T2-hyperintensities of the roots and spinal nerves were rated as pathologic.
Nerve conduction studies (NCS) were performed in all patients at onset and after 6 months according to a predefined algorithm [8]. NCS included median, ulnar, tibial, and fibular nerves on their most involved side including F-waves. In addition, we performed sensory NCS of the sural and the ulnar nerves.
CIDP diagnosis was classified in accordance with EFNS/PNS guidelines [11]. To further quantify NCS data, we used a scoring system, namely the nerve conduction study score (NCSS), which has been described previously by Haertig et al. [8]: “Each pathologic motor nerve is scored with a maximum of 3 points: i) 1 point is assigned for reduced conduction velocity (CV), distal motor latency, prolonged F-wave latency, or possible conduction block; ii) 1 additional point is added if these values reached percentages proposed by EFNS/PNS guidelines [11]; and iii) 1 point is further added for reduced amplitude of the distal compound muscle action potential (CMAP). Complete lack of motor response i scored with the maximum of 3 points. Each pathologic sensory nerve is scored with a maximum of 2 points: i) 1 point for CV reduction and ii) 1 point for reduction of the sensory nerve action potential (SNAP). In analogy to the motor scoring system, 2 points are assigned if no SNAP was evocable. The sum of all points of all these nerves yields the NCSS, with a maximum total score of 16 points. The change of the NCSS (Δ) was calculated by the difference of the NCSSfollow-up − NCSSonset” [8].
High-resolution ultrasound (HRUS) examination (performed on a Mindray 14 MHz TE7) included measurements of the nerve cross-sectional area (CSA) of the median, ulnar, tibial, and fibular nerve (summarized in the UPSA, 16 points); the diameter of the roots C5 and C6 as well as the CSA of the vagus nerve (UPSB, 3 points); and the sural, superficial radial, and fibular nerve (UPSC, 3 points). The exact measurement locations are mentioned in Table 1. The maximum score of the UPSS (UPSA + UPSB + UPSC) is thus 22 points. Finally, longitudinal changes in UPSS were evaluated (UPSSfollow-up – UPSSonset (ΔUPSS)) [8, 14]. Homogeneity of the nerves was evaluated for the median, the ulnar, and the tibial nerve and a maximum of 9 points was reached if all nerves were enlarged homogenously > 1.5-fold (homogeneity score, HS [8]).
Table 1.
Measurement locations for the ultrasound pattern sum score (UPSS)
| UPSA | UPSB | UPSB | |||
|---|---|---|---|---|---|
| Median nerve |
Mid-UA Elbow* Mid-FA |
Cervical root 5 | After leaving processus transversus | Superficial radial nerve | Frohse arcade |
| Ulnar nerve |
Mid-UA Mid-FA |
Cervical root 6 | After leaving processus transversus | Superficial fibular nerve | Distal lateral lower limb |
| Tibial nerve | Popliteal ankle | Vagus nerve | Trigonum iugulare | Sural nerve | Distal calf next to SSV |
| Fibular nerve | Popliteal |
UPSS/A/B/C = ultrasound pattern sum score A/B/C; Mid = middle; UA = upper arm; FA = forearm; SSV = small saphenous vein
*Before entering the pronator muscle. Enlargement > 100% of normal reference value (according to 14 or 12 if you use my reference list) is scored with 1 point, in UPSA enlargement > 150% is scored with 2 points
Additionally, the maximum fascicle size was measured at the level of the upper arm (for median and ulnar nerves) and the knee (for fibular nerve) according to previously published boundary values and fascicular patterns [15]. In analogy to our study and that of Padua et al. concerning echointensity of CIDP patients, we classified nerve morphology as either hypoechoic (class 1) or hyperechoic (class 2) enlarged (UPSS ≥ 5 points) or as not enlarged (UPSS < 5 points, class 3). Echointensity was semi-quantitatively evaluated as described before as predominantly “hypoechoic if the nerve fascicle appearance was comparable to vessel lumen or hyperechoic if comparable to lymph node structure” [8, 16].
Statistics
“t test with Bonferroni correction was used to evaluate differences in epidemiological data (age, height, and weight), clinical scores, ultrasound, and NCS data. To examine the validity of HRUS findings with respect to longitudinal disease development, we correlated ΔUPSS with ΔMRCSS, ΔINCAT, and ΔNCSS as described above (regression analysis and Pearson’s correlation coefficient). Negative values of ΔUPSS, ΔNCSS, and ΔINCAT indicate improvement, whereas a negative ΔMRCSS indicates deterioration and vice versa. Differences in all scores of at least 1 point were rated as improvement or deterioration. The correspondence of cross-sectional UPSS, NCSS, INCAT, and MRCSS and their Δ, the frequency of distinct ultrasound classes (echointensities), and gender were evaluated by cross-table analysis and Fisher’s exact test. Linear regression analysis was also used to test for a linear correlation among distinct classes, UPSS, MRCSS, INCAT, and NCSS. The NCS scoring and the interpretation of the NCS data with regard to EFNS criteria were done by two examiners blinded to the diagnosis (AG and NW), evaluating the measurements including curve analyzing, who did not perform data acquisition” [8]. Receiver operating characteristics curve analysis was used to calculate cut-off values for the distinct ultrasound scores. Sensitivity, specificity, and positive predictive value (PPV) were calculated as recommended. For all tests, a 2-sided p value < 0.05 was regarded as statistically significant. For statistical analysis, we used IBM SPSS Statistics, version 24 (Chicago, IL, USA). The ultrasound analyses have been performed by AG and NW, blinded to the diagnoses. The intrarater and intraclass correlation coefficient (ICC) for the evaluation of echointensity/ultrasound classes was 0.96 and 1.0 respectively. The interrater ICC was 0.9. For fascicle size and CSA, the intrarater ICC was 0.98 and 0.99, for AG and 0.98 and 1.0 for NW respectively, whereas the interrater ICC was 0.96 and 0.99.
Results
Between May 2015 and December 2017, overall 67 patients have been included. Thirty-three of them have been diagnosed as GBS variants (26 AIDP, 3 AMSAN, 3 MFS–GBS, 1 MFS) and 34 as CIDP (28 classical, 5 Lewis–Sumner syndrome, 1 distal acquired demyelinating sensory variant DADS).
First Visit
Clinical Stage I
Overall, the median duration from onset of symptoms to presentation at hospital was significantly higher in CIDP compared with that in GBS (5 weeks, range 1–36 vs 1, 1–12; t test with Bonferroni correction p < 0.001). However, 17 CIDP patients presented with disease duration of no more than 4 weeks (50%), whereas 2 GBS patients presented after more than 12 weeks of symptoms arising. In the latter, however, the maximum of disability was reached in between 8 weeks. Using Fisher’s exact, no significant differences for the gender distribution were found (2.986, p = 0.104). Age, height, and weight did not differ significantly (p = 0.054, 0.092, 0.10). Altogether, clinical stages ranged from severely affected to mild symptoms in both groups according to the clinical scores used. In GBS, the MRC sum score and the INCAT overall disability score revealed significantly higher clinical disability (t test with Bonferroni, p = 0.01 and 0.002) at first presentation in the hospital. MRI of the lumbar spine showed no significant differences of pathology with 8/19 patients with Gd enhancementies/T2-hyperintensities in GBS and 7/21 in CIDP (Fisher’s exact 1.719, p = 0.432). The median CSF-protein values were not significantly different in both groups (t test with Bonferroni, p = 0.631); however, the frequency of pathologic CSF protein was significantly higher in CIDP (27 of 30 patients) compared with that of 19 of 31 patients with GBS (Fisher’s exact 6.777, p = 0.016). Thirteen of 23 GBS patients had positive ganglioside antibodies (1 patient showed NMDA-IgG antibodies) compared with 4 of 20 CIDP patients, although this difference missed significance in Fisher’s exact (13.453, p = 0.385). The clinical data at onset did not significantly differ between the groups with several therapeutic concepts. Two patients received steroids in addition to the intravenous immunoglobulin (IVIG) in GBS due to positive NMDA-IgG found in one and due to concomitant severe asthma in the other. One patient with GBS received steroids alone, who was first treated by our department of internal medicine because of initially suggested graft-versus-host disease. As this patient improved spontaneously, no further IVIG was administered. The detailed data are shown in Table 2.
Table 2.
Patients’ characteristics
| Sex (w/m) | Age | Weight in kg | Height in cm | CSF protein in mg/dl | MRI | MRCSS | INCAT | Antibodies | First therapy | |
|---|---|---|---|---|---|---|---|---|---|---|
| GBS | 6/27 | 53 (3–83) | 75 (18–100) | 175 (110–195) | 67.0 (32–205) | 8/19* | 52 (11–60) | 4 (1–10) |
GM1-IgG n = 3 GM4-IgG n = 2 GQ1b-IgG n = 2 Others n = 7 |
IVIG/PEx n = 24 None n = 1 IVIG-repeat n = 5 IVIG + steroids n = 2 Steroids n = 1 |
| CIDP | 12/22 | 58 (35–88) | 80 (59–125) | 179 (158–198) | 71.5 (34–126) | 7/21 | 57 (36–60) | 3 (1–10) |
GM1-IgG n = 2 Sulfatide n = 1 GM1-IgM n = 1 |
IVIG/Pex n = 5 IVIG-repeat n = 23 IVIG + steroids n = 2 Steroids n = 2 IVIG+IM = 2 |
| p values | 0.104 | 0.054 | 0.092 | 0.100 | 0.631 | 0.432 | 0.01 | 0.002 | 0.385 |
p values < 0.05 are significant
GBS = Guillain–Barré syndrome; CIDP = chronic inflammatory demyelinating polyradiculoneuritis; CSF = cerebrospinal fluid; MRI = magnetic resonance imaging of the lumbar spine; IM = immunosuppressant; G = ganglioside, * = gadolinium enhancement/T2-hyperintensities in 8 of 19 vs. 7 of 21 performed MRI
Nerve Conduction Studies I
In both groups, patients with predominant demyelinating signs in NCS predominated (26 GBS vs 27 CIDP, p = 0.293); thus, comparison of axonal and demyelinating subtypes has been avoided. The occurrence of A-waves was significantly more often associated to GBS (Fisher’s exact 10.211, p = 0.001 with 19/33 in GBS compared with 6/34 positive in CIDP); even eSSP was more often seen in GBS with 24 of 33, but also occurred in 17 of 34 CIDP patients (Fisher’s exact 3.643, p = 0.048). The NCSS at onset did not differ between both groups with median 8 points, range 0 to 13, in CIDP and 6 points, range 0 to 16, in GBS (p = 0.063).
Ultrasound Data I
UPSS
In CIDP and in GBS, overall UPSSonset was enlarged compared with normative UPSS data for healthy controls in literature [14]. However, overall enlargement was significantly higher in CIDP compared with that in GBS in UPSS, UPSA, and UPSC (p < 0.001 each, Fig. 1A, a1), but not in the UPSB (standing for roots and vagus), which showed no significance between both groups (p = 0.313, Fig. 1A, b1). Even after exclusion of all CIDP patients with delayed presentation at hospital (> 4 weeks), these differences were still significant for UPSA, UPSC, and UPSS (t test with Bonferroni, p < 0.001 each compared with GBS). Detailed data are shown in Table 3. None of the GBS patients reached more than 9 points in the UPSS. The UPSB in contrast was enlarged in 32 of 33 patients with GBS and thus was the basis of the overall UPSS enlargement in this group. Albeit the sensory score, UPSC was spared in 30 of 33 GBS patients (further called ultrasound sensory sparing pattern (uSSP)) compared with only 9 of 34 CIDP patients (Fisher’s exact for uSSP 28.127, p < 0.001). uSSP in combination with UPSB increase was found in 29 of 33 GBS patients and in only 5 of 34 CIDP patients, and thus had high accuracy for the diagnosis of GBS (sensitivity 87.8%, specificity 85.3%, PPV 85.3%).
Fig. 1.
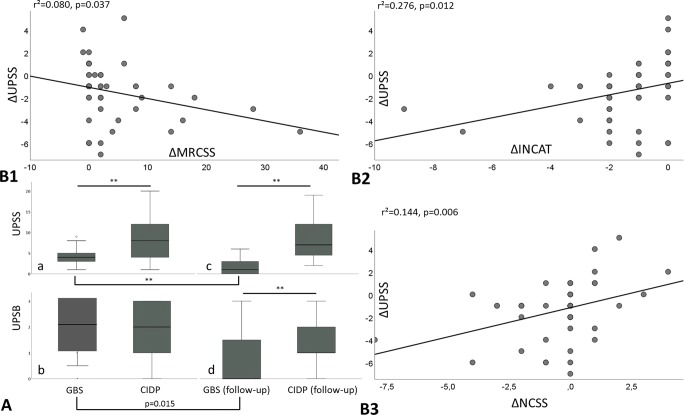
Overall UPSS data at onset (A, a) with significant larger values in CIDP (asterisk symbol) compared to GBS, whereas the UPSB (A, b) is not significantly different. In contrast, after 6 months, both scores are significantly larger in CIDP (A, c and d). Figure B shows the correlation of all CIDP and GBS patients` changes (Δ) of MRCSS (B1), the INCAT overall disability score (B2), and the NCSS (B3) with the ΔUPSS, which correlate all significantly. Patients with the same (Δ)s are summarized as one dot for clarity
Table 3.
Median ultrasound pattern sum score (UPSS) data
| UPSS Median (range) |
UPSA Median (range) |
UPSB Median (range) |
UPSC Median (range) |
|
|---|---|---|---|---|
|
GBS n = 33 |
4, 1–9 | 2, 0–5 | 2, 0–3 | 0, 0–2 |
|
CIDP <4 weeks n = 17 |
7, 1–20 | 4, 0–15 | 2, 0–3 | 1, 0–3 |
|
CIDP >4 weeks n = 17 |
8, 2–22 | 5, 1–16 | 2, 0–3 | 1, 0–3 |
| All CIDP | 8, 1–22 | 5, 0–16 | 2, 0–3 | 1, 0–3 |
| p values* (t test with Bonferroni) |
0.001 0.001 0.001 1.0** |
0.001 0.001 0.001 1.0** |
1.0 0.427 0.313 0.795** |
0.001 0.001 0.001 0.231** |
All CIDP patients, even the subgroups consisting of those with less and those with more than 4 weeks of symptoms, had significantly larger values for UPSA, UPSC, and UPSS, but not for UPSB
GBS = Guillain–Barré syndrome; CIDP = chronic inflammatory demyelinating polyradiculoneuritis
*Comparison of GBS to CIDP < 4 weeks, > 4 weeks, and all CIDPs (p values < 0.05 are significant; nonsignificant differences are in italic). **Between CIDP < 4 weeks and > 4 weeks, no significant differences occur
In contrast, sensory nerve enlargement (UPSC > 0) revealed high specificity (90.9%) and PPV (88.9%) for CIDP in the ROC curve analysis (AUC = 0.825, p < 0.001, sensitivity 66.7%). Similarly, peripheral nerve enlargement in more than 3 sensorimotor landmarks (UPSA > 3) was significantly correlated with CIDP (AUC = 0.825, sensitivity 66.7%, specificity 82.8%, PPV 78.8%) (Fig. 2A).
Fig. 2.
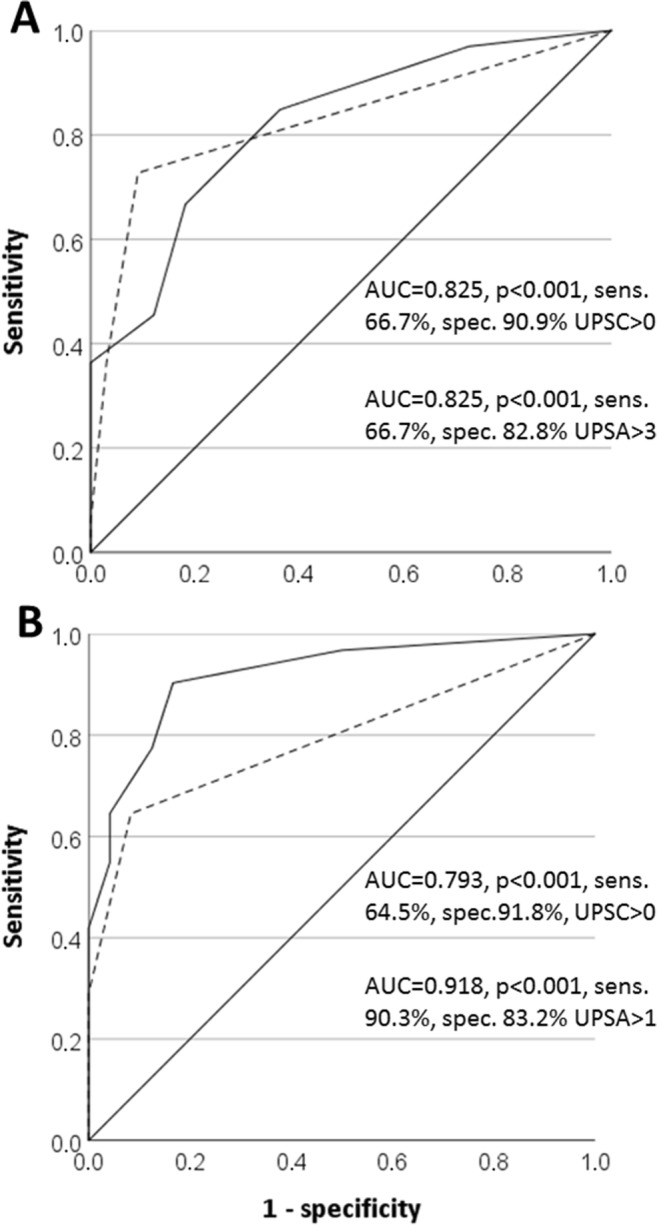
ROC curve analysis for UPSA (drawn line) and UPSC (dotted line) at onset (A) and after 6 months (B) to differentiate CIDP from GBS with high sensitivity and specificity in both curves
Fascicles, Homogeneity, and Echointensity
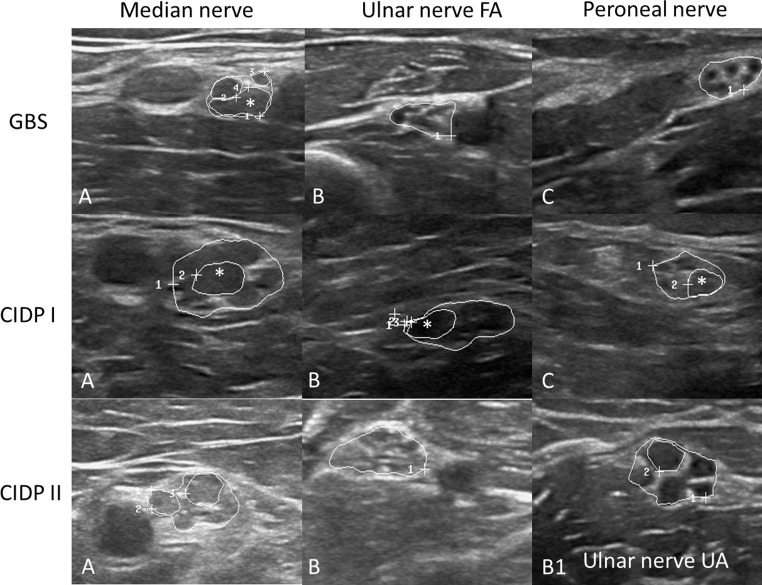
In most patients with GBS, fascicles were not or only regionally restricted enlarged, whereas in CIDP, a significantly more heterogeneous distribution of fascicle pattern was found ranging from no enlargement to diffuse enlargement affecting most fascicles measured (Fisher’s exact 9.378, p = 0.008). Further, the HS was significantly enlarged in CIDP (1, range − 3 to 9 vs − 1, − 3 to 4, p < 0.01); nevertheless, the HS revealed mostly regionally or inhomogeneously enlarged nerves with proximal predominance in both entities. All patients with GBS revealed hypoechoic class 1 (33.3%) or class 3 pattern (66.6%), whereas in CIDP, all three classes were presented, mostly class 1 pattern (52.9%), but also hyperechoic class 2 (20.6%), or class 3 pattern (26.5%) (Fisher’s exact for distinct echointensity frequencies 14.315, p < 0.001). Figure 3 gives an overview of the fascicle pattern, echointensity, and homogeneity in GBS and CIDP examples.
Fig. 3.
Examples of median (A), ulnar (B), and fibular/peroneal nerves (C) in one GBS (first row) and two CIDP patients. In the GBS patient, the echointensity of the fascicles is hypo- to isoechoic, only the median nerve (MN) is slightly enlarged—only in the upper arm—regionally restricted (13 mm2, cut-off 12 mm2), whereas ulnar and fibular nerves (UN and FN) are not enlarged. Only one fascicle is enlarged (*, MN, CSA 7 mm2, cut-off, 4.8 mm2, 15). In the CIDP I patient in contrast, all nerves are enlarged to a different amount (MN 45 mm2, the UN 26 mm2, cut-off 8.5 mm2 and the FN 18 mm2, cut-off 11.5 mm2). In all nerves, at least one fascicle is enlarged (* 10 mm2 in the MN, 6 and 7 mm2 in UN and FN, cut-off 2.8 and 3.5 mm2). In CIDP II, the MN is enlarged with 25 mm2 and reveals increased echointensity, and the UN is enlarged at two sites—homogeneously (18 mm2 in the upper arm (B1) with reduced echo signal and 13 mm2 in the forearm with increased echo signal (B)). Nerves are surrounded by white circles; the mentioned fascicles are marked with asterisk symbols
Correlation of Clinical, Electrophysiological, and Ultrasound Data I
Nerve enlargement (UPSS) did not correlate with motor or sensory impairment neither in GBS (MRCSS/INCAT: r2 = 0.006, p = 0.682 and r2 = 0.016, p = 0.414) nor in CIDP (r2 = 0.056, p = 0.178 and r2 = 0.057, p = 0.212). Even NCSS and clinical scores did not correlate in all groups (r2 = 0.015 and 0.021, p = 0.453 and 0.345 for GBS and r2 = 0.032 and 0.043, p = 0.656 and 0.321 for CIDP). Further, NCSS and UPSS correlated only by trend (r2 = 0.313, p = 0.080). 23 of 24 patients with GBS and electrophysiological eSSP also showed uSSP. Seven patients with uSSP omitted eSSP. In contrast, in CIDP, only 5 of the 17 patients with eSSP exhibited additional uSSP (Fisher’s exact, p < 0.001, 20.183). The combination of the electrophysiological sural and ultrasonic sensory nerve sparing pattern therefore showed a positive predictive value for the diagnosis of GBS in 82.2% with a sensitivity of 69.9% and a specificity of 85.3%.
Six-Month Follow-up Data II
Overall, 12 patients (9 with GBS [27.2%] and 3 with CIDP [8.8%]) did not get a follow-up visit due to death of other reasons (n = 2), by missing or by refusing the second examination (n = 10).
Clinical Stage II
In both groups, most patients remained stable or even improved after 6 months (rate of improvement, 50% in CIDP and 62.9% in GBS for MRC increase and 58.2% vs 85.7% for INCAT decrease). The rate of patients with significant improvement was higher in the GBS group (Fisher’s exact, p < 0.0001 for INCAT and p = 0.048 for MRCSS). The median improvement consequently was significantly higher in GBS than that in CIDP (ΔMRCSS + 3, range 0–36 compared with 0, range 1–8, p = 0.002; ΔINCAT − 2, range −9 to 0 compared with − 1, range − 2 to 0, p < 0.001). The amount of improvement did not differ between the distinct therapeutic groups.
Nerve Conduction Studies II
The NCSSfollow-up significantly decreased in GBS compared with CIDP patients (median, 3, 0–15 and 9, 0–14; p = 0.002, t test with Bonferroni), in which the median NCSS data remained almost unchanged compared with the NCSSonset (p = 0.909, t test with Bonferroni correction).
Ultrasound Data II
Fourteen (58.3%) GBS patients showed less than 2 points left in UPSS, and 9 GBS patients (> 30%) already revealed overall normal nerves. None had more than 6 points. In contrast, more than 50% of CIDP patients had more than 6 points in the UPSS; none recovered completely. Overall, median UPSSfollow-up decreased to 1 point in GBS, range 0 to 6 (p < 0.001 compared with UPSSonset), whereas in CIDP, the median UPSSfollow-up reached 7 points, range 2 to 19 (compared with 8 points at onset, p = 0.909) and thus was still significantly larger than in GBS patients (p < 0.001, Fig. 1A, c). However, in contrast to the onset of disease, CIDP patients now reached significantly larger values in all sub-scores (UPSAfollow-upp < 0.001, UPSBfollow-upp = 0.02, and UPSCfollow-upp < 0.001, Fig. 1A, d). The median total change of UPSS (UPSSonset − UPSSfollow-up) was significantly higher in GBS than that in CIDP (p = 0.045); also, the rate of patients with UPSS decrease was significantly higher in GBS (n = 21 of 24) compared with that in CIDP (n = 18 of 31), Fisher’s exact 5.682, p = 0.020. Comparable with first visit data, a positive UPSC and a cut-off value of > 1 point in the UPSA after 6 months were highly associated with the diagnosis of CIDP (AUC = 0.793/0.918, p < 0.001, sensitivity 64.5/90.3%, specificity 91.8/83.2%) (Fig. 2B).
Correlation of Clinical, Electrophysiological, and Ultrasound Data II
Equivalently to the onset data, nerve enlargement (UPSS) did not correlate with motor or overall impairment neither in both groups (r2 = 0.03, p = 0.645 in GBS and r2 = 0.006, p = 0.756 in CIDP) nor in the subgroups (values not shown due to clarity). Even NCSS and clinical scores do not correlate in all groups (r2 = 0.018, p = 0.312 and 0.31, p = 0.201). However, NCSS and UPSS correlate significantly (r2 = 0.148, p = 0.006).
Moreover, the ΔUPSS significantly correlated with the ΔINCAT (r2 = 0.276, p = 0.012) and inversely with the ΔMRCSS (r2 = 0.080, p = 0.037) (Fig. 1B 1–2). Additionally, ΔNCSS and ΔUPSS correlate (r2 = 0.144, p = 0.006) (Fig. 1B 3). Figure 4 gives an example of GBS and CIDP patients before and 6 months after therapy.
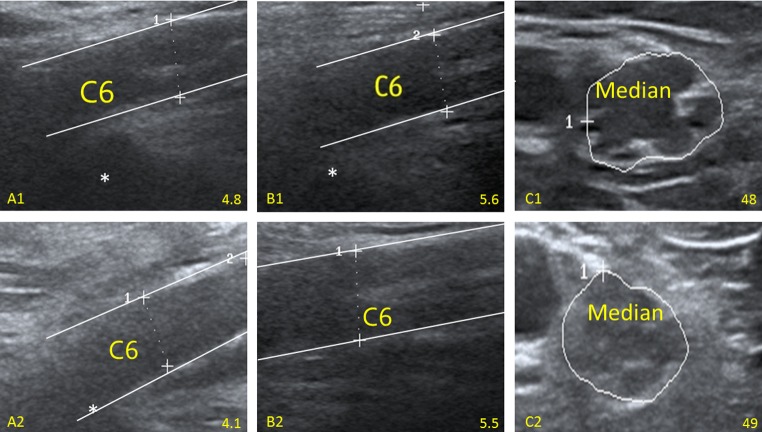
Fig. 4.
Examples of a GBS and a CIDP patient before and 6 months after therapy. A1 and A2 reveal the course in a GBS patient with a significant decrease of nerve root enlargement (cervical root 6, C6; 4.8 to 4.1 mm, reference < 4.2 mm, according to 14) compared with a CIDP patient. Here, the cervical root 6 (B1 and B2 5.6 and 5.5 mm) and the median nerve (C1 and C2, 48 mm2 and 49 mm2, reference < 12 mm2, 14) are similarly enlarged in both examinations
Discussion
Treatment options for immune-mediated neuropathies differ depending on subtype. CIDP patients mostly need ongoing, repeated therapies with either IVIG or steroids, whereas in GBS—due its mostly monophasic course—IVIG once is sufficient and steroids regularly do not help. Therefore, a distinction of both—sometimes similarly starting—entities is crucial.
Nerve enlargement is the most intriguing finding in immune-mediated neuropathies in ultrasound, in acute as well as in chronic types. Whereas in CIDP, this enlargement emerges in peripheral nerves, even on a fascicular level, in the pure sensory nerves and in the roots and the vagus nerve, in GBS, this enlargement is found particularly in the roots and the vagus nerve.
Thus, in GBS, the finding of multiple A-waves, the electrophysiological sural sparing next to ultrasonic sensory sparing pattern (uSSP), and swelling of the roots and vagus nerve are diagnostic hallmarks. In contrast, CIDP outstandingly reveals enlarged sensory nerves and/or multifocally enlarged peripheral nerves (UPSA > 3 points as threshold); further, increased echointensity (class 2)—if arising—seems to be a specific distinguishing feature for CIDP (specificity 100%), even in short-dated disease course. After 6 months, nerve enlargement in GBS often disappears or at least decreases significantly, whereas in CIDP, overall nerve enlargement remains—with heterogeneous development—almost unchanged. The longitudinal clinical process is well resembled by the ultrasound behavior in both groups.
Nerve enlargement is a commonly described finding in immune-mediated neuropathies with a heterogeneous and regionally predominant pattern, particularly in arm nerves and roots in CIDP and variants and marked nerve root and vagus nerve enlargement in GBS [5–7, 16–19]. By defining scores, i.e., the UPSS or the Bochum ultrasound score, a distinction between acute and chronic variants was discussed several times [5, 6]. For CIDP, a significant correlation of ultrasonic and clinical course over 12 or more months has been described recently, whereas in GBS, decrease of nerve enlargement over less than 6 months has been shown [8–10, 20, 21]. This, to the best of our knowledge, is the first study describing ultrasound, electrophysiological, and clinical data comparatively over a predefined period of 6 months.
In our study group, next to the GBS patients, even 50% of the CIDP patients presented with symptom onset of less than 4 weeks and thus could have been clinically misdiagnosed at first visit. Patients with GBS showed higher motor and sensory impairment compared with CIDP patients in our group. MRI of the lumbar spine or ganglioside antibody data showed no significant differences and thus could not contribute for distinction. More patients in the CIDP group showed overall CSF pathology—as in GBS, CSF can often be normal during the first days [4]—however, the median protein elevation did not significantly differ.
NCS data showed demyelinating pathology in most patients, either in GBS as in CIDP. Nevertheless, in GBS, the presence of A-waves was seen more often than in CIDP (p = 0.001). Similarly, eSSP in NCS was more often seen in GBS, but also emerged in 50% of CIDP patients (p < 0.05). The amount of electrophysiological nerve pathology as measured by the NCSS did not differ significantly.
In ultrasound, both CIDP and GBS show root and vagus nerve enlargement (UPSB) in the first weeks of disease, whereas in CIDP, enlargement of the peripheral nerve segments and particularly pure sensory nerves is significantly marked compared with GBS (UPSA and UPSC, p < 0.001). This finding is in line with previous results [5, 6].
As a new finding, we could demonstrate these different patterns also in acute-onset CIDP patients with less than 4 weeks of symptom onset (p < 0.001 for UPSA, UPSC, and UPSS compared with GBS). With regard to the echointensity, in all GBS patients, echointensity was reduced (class 1 or class 3), whereas in CIDP, hyperechoic echointensity was already seen in > 20% of cases. Nevertheless, we must consider that hypoechoic nerve enlargement as a possible sign of edema [8] predominates in early stages of disease.
As pathognomonic for GBS patients, we could therefore highlight sensory nerve enlargement sparing in ultrasound (uSSP) in most of our patients in contrast to CIDP; herein, pure sensory nerves were mostly regionally enlarged. A combination of ultrasound and electrophysiological SSP or a combination of UPSB increase and uSSP was a key feature for the diagnosis of GBS (sensitivity ~ 70% to > 85%, specificity > 85%, and PPV > 82% to > 85% respectively).
In contrast, sensory nerve enlargement (UPSC > 0) and/or obvious sensorimotor nerve enlargement (UPSA > 3) were hallmarks of CIDP (sensitivity 66.7%, specificity 82.8–100%, and PPV 82.8–100%), whereas increased echointensity has an excellent PPV and specificity of 100% for CIDP if arising at early stage.
Cross-sectional comparison of clinical, ultrasound, and electrophysiological data has no suitable correlation; this is in line with previous data [6, 8, 10, 16].
After an observational period of 6 months, both groups showed clinical improvement in many patients; however, the median amount of improvement—as measured by MRCSS and INCAT overall disability score—and the intraindividual relative improvement were significantly higher in GBS patients. The NCSS improved significantly in GBS patients, whereas it remained overall unchanged in CIDP. Accordingly, decrease of nerve enlargement was significant in GBS with almost normalization in up to 60% of patients and only slight nerve enlargement left in the others. In contrast, all CIDP patients showed persisting nerve enlargement, and more than 50% of these patients even showed significant nerve enlargement (p < 0.001 compared with GBS) with more than 6 points in the UPSS; the rate of patients with improved UPSS was thus significantly higher in GBS than that in CIDP (p = 0.020). Persisting peripheral sensorimotor (UPSA > 1 point) or pure sensory nerve enlargement (UPSC > 0) over 6 months was still significantly associated with a chronic type of immune-mediated neuropathy, the UPSA with an excellent sensitivity > 90%, and the UPSC with high specificity > 90%. In contrast to the cross-sectional data, the longitudinal development of ultrasound, clinical disability, and NCS (evaluated as delta) correlates significantly with each other, which means the better the clinical recovery is, the larger the decrease of nerve enlargement is and vice versa. This is in line with previous data of Härtig et al. and Zaidman et al. for CIDP [8, 10].
In contrast to the work of Härtig et al. [8], no significant UPSS decrease is seen in CIDP, which might be due to the shorter observational period. Therefore, ultrasound, NCS, and clinical score might serve as follow-up tool over a longer disease course in CIDP, whereas in GBS, early decrease of nerve enlargement is a common finding [9].
As a limitation we must consider, altogether 12 patients (27.2% of GBS and 8.8% of CIDP patients) got lost to follow-up visit, which might have biased the data. Further, CIDP patients have been older by trend than GBS patients; this fact might also have an influence on the UPSS data as elder people tend to have larger nerves. However, significant differences have only been described for few sites of the UPSS [14].
Our study demonstrates that in CIDP, nerve enlargement and clinical impairment persist longer than in GBS. This difference might be a consequence of the more acute nerve pathology in GBS (edema, inflammation, and thus conduction block) compared with the more chronic damage in CIDP (next to edema and inflammation, pronounced fibrosis and onion bulb formation as well as long distance and multifocal demyelination and axonal damage), which might already arise in early CIDP. The pathognomonic occurrence of class 2 pattern in CIDP might be a further proof of this assumption as increased echosignal might be a consequence of chronic nerve damage [8]. These findings might help to discontinue or to continue therapy in cases, in which differentiation might be difficult, if nerve morphology has overall normalized after 6 months or significant enlargement might still arise. Ultrasound might herald the start of a new era of immunotherapy in neuropathies [22].
Conclusion
Ultrasound in combination with electrophysiology facilitates the early differentiation between GBS and acute-onset CIDP and serves as an observational tool, whereas CSF analysis and antibody testing do not contribute to differentiation. As GBS patients show nerve root and vagus enlargement at the beginning in combination with A-waves, and ultrasonic sensory sparing as well as electrophysiological sural sparing pattern, in CIDP, multifocal peripheral nerve enlargement and enlargement of pure sensory nerves seem to be a hallmark; increased echointensity is only found in CIDP. Further, ultrasound and NCS might be a resource, which could influence ongoing therapeutic steps. After 6 months, in GBS, nerve morphology normalizes and NCSS improves, whereas in CIDP, morphological and functional nerve pathology might persist more rigidly. These tools therefore might facilitate therapeutic release or continuity.
Electronic Supplementary Material
(PDF 489 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- AIDP
acute inflammatory demyelinating polyradiculoneuritis
- AM(S)AN
acute motor (and sensory) neuropathy
- C5 and 6
cervical roots 5 and 6
- CIDP
chronic inflammatory demyelinating polyradiculoneuritis
- CMAP
compound muscle action potential
- TN
treated chronic neuritis
- CSA
cross-sectional area
- CSF
cerebrospinal fluid
- EDx
electrodiagnostics
- EFNS
European Federation of Neurological Societies
- GBS
Guillain–Barré syndrome
- HRUS
high-resolution ultrasound
- INCAT
inflammatory neuropathy cause and treatment disability score
- IVIG
intravenous immunoglobulin
- MADSAM
multifocal acquired demyelinating sensory and motor neuropathy
- MFS
Miller–Fisher syndrome
- MRCSS
medical research council sum score
- NCS
nerve conduction studies
- NCSS
NCS score
- PPV
positive predictive value
- RTX
rituximab
- UPSS
ultrasound pattern sum score
Authors’ Contributions
Hannah Oertl, Natalie Winter, and Alexander Grimm designed the study and participated in data acquisition, analysis, interpretation, and drafting of the manuscript. Eva Auffenberg, Christoph Ruschil, Hubertus Axer, and Victoria Schubert participated in data acquisition and were involved in drafting the manuscript. All authors read, critically revised, and approved the final manuscript.
Funding Information
AG received reimbursement for travel and accommodation costs from Pfizer for a polyneuropathy symposium. AG and NW received grant from German Ultrasound Society (DEGUM).
Compliance with Ethical Standards
The study was registered in the German clinical trial registry (DRKS-ID 00005253) and approved by the local ethics committee (Tübingen 702/2015BO2). Written informed consent to study participation was obtained from all patients. The diagnosis of immune-mediated neuropathy was done by EFNS guidelines as well as according to Asbury et al. [5, 11] as recommended.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol. 2014;10:435–446. doi: 10.1038/nrneurol.2014.117. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat. Rev. Neurol. 2011;7:507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- 3.Asbury AK. Diagnostic considerations in Guillain-Barré syndrome. Ann Neurol. 1981;9(Suppl):1–5. doi: 10.1002/ana.410090703. [DOI] [PubMed] [Google Scholar]
- 4.Ruts L, Drenthen J, Jacobs BC, van Doorn PA. Dutch GBS Study Group. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology. 2010;74(21):1680–6. doi: 10.1212/WNL.0b013e3181e07d14. [DOI] [PubMed] [Google Scholar]
- 5.Grimm A, Decard BF, Axer H, Fuhr P. The Ultrasound pattern sum score - UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol. 2015;126(11):2216–2225. doi: 10.1016/j.clinph.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Bochum ultrasound score versus clinical and electrophysiological parameters in distinguishing acute-onset chronic from acute inflammatory demyelinating polyneuropathy. Muscle Nerve. 2015;51(6):846–52. doi: 10.1002/mus.24484. [DOI] [PubMed] [Google Scholar]
- 7.Zaidman CM, Harms MB, Pestronk A. Ultrasound of inherited vs. acquired demyelinating polyneuropathies. J Neurol. 2013;260(12):3115–3121. doi: 10.1007/s00415-013-7123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Härtig F, Ross M, Dammeier NM, Fedtke N, Heiling B, Axer H, Décard BF, Auffenberg E, Koch M, Rattay TW, Krumbholz M, Bornemann A, Lerche H, Winter N, Grimm A. Nerve Ultrasound Predicts Treatment Response in Chronic Inflammatory Demyelinating Polyradiculoneuropathy-a Prospective Follow-Up. Neurotherapeutics. 2018;15(2):439–451. doi: 10.1007/s13311-018-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm A, Décard BF, Schramm A, Pröbstel AK, Rasenack M, Axer H, Fuhr P. Ultrasound and electrophysiologic findings in patients with Guillain-Barré syndrome at disease onset and over a period of six months. Clin Neurophysiol. 2016;127(2):1657–1663. doi: 10.1016/j.clinph.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Zaidman CM, Pestronk A. Nerve size in chronic inflammatory demyelinating neuropathy varies with disease activity and therapy response over time: a retrospective ultrasound study. Muscle Nerve. 2014;50(5):733–738. doi: 10.1002/mus.24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joint Task Force of the EFNS and the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 12.Merkies IS, Schmitz PI, van der Meché FG, Samijn JP, van Doorn PA; Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) group. J Neurol Neurosurg Psychiatry. 2002 May;72(5):596–601. [DOI] [PMC free article] [PubMed]
- 13.Panaite PA, Renaud S, Kraftsik R, Steck AJ, Kuntzer T. Impairment and disability in 20 CIDP patients according to disease activity status. J Peripher Nerv Syst. 2013;18(3):241–6. doi: 10.1111/jns5.12038. [DOI] [PubMed] [Google Scholar]
- 14.Grimm A, Axer H, Heiling B, Winter N. Nerve ultrasound normal values - Readjustment of the ultrasound pattern sum score UPSS. Clin Neurophysiol. 2018;129(7):1403–1409. doi: 10.1016/j.clinph.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Grimm A, Winter N, Rattay TW, Härtig F, Dammeier N, Auffenberg E, et al. A look inside the nerve –morphology of nerve fascicles in healthy controls and patients with polyneuropathy. Clin Neurophysiol 2017c (accepted) [DOI] [PubMed]
- 16.Padua L, Granata G, Sabatelli M, Inghilleri M, Lucchetta M, Luigetti M, et al. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol. 2014;125(1):160–165. doi: 10.1016/j.clinph.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Grimm A, Vittore D, Schubert V, Lipski C, Heiling B, Decard BF, et al. Ultrasound pattern sum score, homogeneity score and regional nerve enlargement index for differentiation of demyelinating inflammatory and hereditary neuropathies. Clin Neurophysiol. 2016;127(7):2618–2624. doi: 10.1016/j.clinph.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology. 2015;84(8):803–809. doi: 10.1212/WNL.0000000000001291. [DOI] [PubMed] [Google Scholar]
- 19.Grimm A, Vittore D, Schubert V, Rasenack M, Decard BF, Heiling B, et al. Ultrasound aspects in therapy-naive CIDP compared to long-term treated CIDP. J Neurol. 2016;263(6):1074–1082. doi: 10.1007/s00415-016-8100-9. [DOI] [PubMed] [Google Scholar]
- 20.Fisse AL, Pitarokoili K, Trampe N, Motte J, Kerasnoudis A, Gold R, Yoon MS. Clinical, Sonographic, and Electrophysiologic Longitudinal Features of Chronic Inflammatory Demyelinating Polyneuropathy. J Neuroimaging. 2018 Nov 8. doi: 10.1111/jon.12579 [DOI] [PubMed]
- 21.Kerasnoudis A, Pitarokoili K, Gold R, Yoon MS. Nerve Ultrasound and Electrophysiology for Therapy Monitoring in Chronic Inflammatory Demyelinating Polyneuropathy. J Neuroimaging. 2015;25(6):931–9. doi: 10.1111/jon.12279. [DOI] [PubMed] [Google Scholar]
- 22.Décard BF, Pham M, Grimm A. Ultrasound and MRI of nerves for monitoring disease activity and treatment effects in chronic dysimmune neuropathies - Current concepts and future directions. Clin Neurophysiol. 2018;129(1):155–167. doi: 10.1016/j.clinph.2017.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 489 kb)