Table 6.
Calculation of the formation enthalpies of the studied substances using the addictive Benson scheme for 5-nitrophenyl-2-furaldehyde oxime isomers
| Substance | № | Increment | ΔfH298.15, kJ mol−1 |
|---|---|---|---|
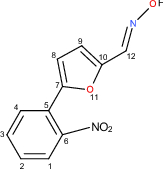
|
1–4 | Cb–(Cb)2(H) | 13.8 |
| 5 | Cb–(Cb)2(Cd) | 23.8 | |
| 6 | Cb–(Cb)2(NO2) | − 0.5 | |
| 7 | Cd–(Cd)(Cb)(O) | 59.7 | |
| 8,9 | Cd–(Cd)2(H) | 28.4 | |
| 10 | Cd–(Cd)2(O) | 43.4 | |
| 11 | O–(Cd)2 | − 137.2 | |
| 12 | Cd–(Cd)(H)(N–OH)* | 33.0 | |
| Furan cycle | − 25.9 | ||
ΔfH298,15 = 4·ΔfH298,15(Cb–(Cb)2(H)) + ΔfH298,15(Cb−(Cb)2(Cd)) + ΔfH298,15(Cb–(Cb)2(NO2)) + ΔfH298,15(Cd–(Cd)(Cb)(O)) + 2·ΔfH298,15(Cd–(Cd)2(H)) + ΔfH298,15(Cd–(Cd)2(O)) + ΔfH298,15(O–(Cd)2) + ΔfH298,15(Cd–(Cd)(H)(N–OH)) + ΔfH298,15(Furan cycle) = 4·13.8 + 23.8 − 0.5 + 59.7 + 2·28.4 + 43.4 − 137.2 + 33.0 − 25.9 = 108.3 kJ/mol
