Abstract
Background
This study aimed to investigate the risk factors for mechanical failure of cement spacers and the impact on hip function after two-stage exchange arthroplasty for periprosthetic joint infection (PJI).
Methods
Thirty-one patients (19 males and 12 females) with hip PJIs underwent resection arthroplasty and implantation of cement spacers from January 2014 to December 2015. Patients who encountered spacer-associated mechanical complications in the interim period (14 of 31) were compared with those without complications (17 of 31). Complications were defined as spacer dislocation, spacer fracture, spacer fracture with dislocation, and femoral fracture during or following spacer implantation. Hip functional outcome was assessed using the Harris hip score (HHS). Treatment success was defined according to the following criteria: (1) no symptoms or signs indicative of infection; (2) no PJI-related mortality; and (3) no subsequent surgical intervention for infection after reimplantation surgery. Multivariate logistic regression and Kaplan-Meier survival curves were used for analysis.
Results
Fourteen patients (14/31 = 45%) suffered at least one spacer-related complication within the interim period. The development of spacer complications was associated with a younger age (odds ratio [OR] 0.91, 95% confidence interval [CI] 0.83–1.00, p = 0.045) and chronic PJI (OR 14.7, 95% CI 1.19–182, p = 0.036). Patients with spacer complications also had a lower median HHS (37 vs. 60, p < 0.001) before reimplantation in comparison to those without spacer complications. After reimplantation, the two groups had a similar median HHS (90 vs. 89, p = 0.945). Two patients did not undergo reimplantation due to extensive comorbidities, and subsequently retained the antibiotic spacer for definitive treatment. The 2-year treatment success rate was 84.6% in the spacer-complication group and 87.5% in the non-spacer-complication group (p = 0.81).
Conclusion
There was a high complication rate for articulating PMMA spacers during the interim period of two-stage revision total hip arthroplasty. A young age and chronic infection were the primary risk factors associated with mechanical complications. Patients at high risk of spacer-related mechanical complications should be advised accordingly by surgeons. Knowing the possible risk factors, surgeons should educate patients thoroughly to avoid spacer complications, thereby increasing patient satisfaction in the interim stage.
Level of evidence
Prognostic Level III.
Keywords: Hip, PMMA, Articulating spacer, Complications, Periprosthetic joint infection
Background
Periprosthetic joint infection (PJI) is a rare but devastating complication of total hip arthroplasty (THA). The gold standard for treating PJI after THA is via two-stage revision arthroplasty, which involves initial removal of the prosthesis and insertion of an articulating polymethylmethacrylate (PMMA) spacer. The second stage of reimplantation consists of insertion of a new prosthesis once the underlying infection has been eliminated [1, 2]. There are several kinds of antibiotic-loaded cement used between stages that have led to favorable success rates, ranging from 82 to 96% [3–8].
Although antibiotic cement beads provide a high local concentration of antibiotics [9], they present numerous disadvantages, including joint instability, increased energy demand during gait, soft-tissue contractures and abnormal stress on the spine from pseudoarthrosis [3, 9]. Articulating PMMA spacers have been shown to eliminate infection at a rate similar to cement beads [4]; however, articulating spacers are advantageous in comparison with beads, in that they provide temporary soft tissue tension and improved hip scores, and allow for increased functional capacity [2]. Additionally, application of an articulating spacer is relatively straight-forward during reimplantation surgery [7], and results in a lower dislocation rate postoperatively in comparison to antibiotic beads [4]. In circumstances in which the patient’s condition does not allow for revision surgery, the articulating PMMA spacer can remain implanted for definitive treatment [10, 11]. In comparison, preformed spacers provide similar infection eradication rates to articulating spacers, as well as good mechanical stability [3, 12]; however, the cost of preformed spacers is much higher than that of articulating PMMA spacers.
At times, mechanical complications may occur following spacer insertion, leading to reduced patient function and mobility. In the first stage, complication rates associated with articulating spacers have been reported to be anywhere from 19.5 % to 50 %[13, 14]. These complications include spacer dislocation, spacer fracture and femoral fracture [15]. To date, it remains unclear whether spacer-associated complications influence the functional outcome following reimplantation. This study aimed to (1) investigate the risk factors for mechanical complications of articulating PMMA spacers, and (2) examine the association between complications arising following implantation of an articulating PMMA spacer and functional outcome following reimplantation. We hypothesized that mechanical complications associated with articulating PMMA spacers might influence the overall functional outcome following reimplantation.
Methods
Patient enrollment
Following Institutional Review Board approval, patients of a single institution who underwent two-stage exchange arthroplasty were enrolled in this study between January 2014 and December 2015. The indications for the two-stage procedure were chronic PJI, acute infection with failed debridement, and implant loosening. Acute infection was defined as occurring within 3 weeks of the procedure, or a late hematogenous infection with development of symptoms within 3 weeks of surgery. Chronic PJI was defined as any infection developing after 3 weeks following surgery [16]. All patients who underwent articulating PMMA spacer implantation were included. The exclusion criteria included any patient who underwent PJI treatment with cement beads, treatment with static spacers, one-stage revision, debridement with or without modular exchange, amputation, or arthrodesis. Patients with megaprosthesis and a history of septic arthritis in the native joint were also excluded. A total of 35 patients who underwent implantation of an articulating PMMA spacer were included in this study initially. One patient was lost to follow-up 4 months after resection arthroplasty, and three patients died from septic shock (unrelated to PJI) within 6 months of resection arthroplasty. Finally, 31 patients (19 men, 12 women) were divided into two groups, those with (group A, n = 14) and without spacer-related complications (group B, n = 17) (Fig. 1). Patients in both groups were followed-up for a minimum of 2 years after reimplantation.
Fig. 1.
Flowchart of eligible patients
Medical charts were manually reviewed to obtain information relevant to the clinical course following resection arthroplasty, and the following measures and data were recorded: patient characteristics, American Society of Anesthesiologists (ASA), prosthesis type, comorbidities, classification of PJI (acute or chronic) [16], serologic markers, interval between resection arthroplasty and reimplantation, surgical approach, bone defects, and microorganism cultures.
Treatment protocol
The first stage consisted of prosthesis removal and radical debridement, followed by temporary articulating spacer placement. A minimum of three sets of tissue specimens were taken for culture, and custom-made silicon molds were used to model the femoral cement spacer (Fig. 2a). Antibiotic-loaded bone cement was added to the silicon mold with two or three 3-mm Kirschner wires forming the endoskeleton. The cup spacer was shaped using a unipolar cup (Fig. 2b). Local antibiotics in the PMMA bone cement were tailored to the organism previously identified by culture. If the infecting organism was not known at the time of resection arthroplasty, broad coverage with vancomycin and ceftazidime was employed. The antibiotic dose per batch of cement (40 g) was limited to 6–8 g.
Fig. 2.
Images showing: a) the femoral and b) the cup part of a PMMA articulating spacer
The cup part of the articulating PMMA spacer was cemented onto the acetabulum, and the femoral part of the articulating spacer was inserted into the femoral canal, with cement added to the meta-diaphyseal portion of the stem. This enhanced construct fixation to prevent spacer dislocation and decrease the risk of cement incarceration in the femoral canal.
Following the first stage of surgery, a physical therapist was consulted for strengthening on the first postoperative day and protected non-weight-bearing activity on the second postoperative day. Toe-touch weight-bearing was allowed 4–6 weeks after the first stage of surgery. Hip precautions were advised for 6 weeks postoperatively to prevent hip dislocation. Organism-specific antibiotics were administered intravenously for 2 weeks after resection arthroplasty, followed by oral antibiotics for 4 weeks. The serum C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) were routinely checked every 2 weeks. Reimplantation was subsequently performed based on the quality of healing of the surgical wound and the following criteria being met: no fistula formation, no drainage, and no elevation of the ESR or CRP level following an antibiotic-free interval of at least 2 weeks. Three sets of deep aerobic and anaerobic bacterial cultures were obtained as routine at the second-stage surgery.
Post-operative empirical antibiotics were prescribed after the second-stage surgery, using the same agent as prior to second-stage surgery, and discontinued after confirmation of a negative culture. Intravenous and oral antibiotics were prescribed for a total of 6 weeks if the culture was positive at the second stage. All the patients undergoing reimplantation were followed-up for a minimum period of 2 years, until death or recurrent infection.
Outcome measurement
Mechanical complications associated with articulating spacers were defined as spacer dislocation, spacer fracture, spacer fracture with dislocation, and femoral fracture during spacer insertion or during the interim period. Hip function was accessed using the Harris hip score (HHS). Treatment success was defined according to the Delphi consensus criteria proposed by Diaz-Ledezma et al. [17]: (1) infection eradication was characterized by a healed wound without drainage, fistula, or pain, and with no recurrence of infection; (2) no occurrence of PJI-related mortality (e.g., sepsis, necrotizing fasciitis); and (3) no subsequent surgical intervention for infection after reimplantation surgery.
Statistical analysis
Statistical analysis was performed using MedCalc (version 17.9.2, Ostend, Belgium). Categorical variables were expressed as counts and percentages, and continuous data as medians and interquartile ranges (IQRs). As a preliminary Kolmogorov-Smirnov test did not demonstrate a normal distribution for our small sample size, we decided to use the nonparametric Mann-Whitney U test to compare the continuous variables. Categorical variables were compared using the chi-square test or the Fisher exact test. Multivariate analysis with binary logistic regression was used to determine further risk factors associated with the development of spacer-related complications (p < 0.10 in the univariate analysis). A Kaplan-Meier survivorship curve was generated to estimate the proportion of patients in whom treatment was successful in the two groups. The difference in survivorship between the two groups was assessed using the log-rank test. A p-value<0.05 was considered statistically significant.
Results
The median age of the whole cohort of 31 patients was 56 years (interquartile range, IQR: 50–71). The median body mass index (BMI) was 23.7 kg/m2 (IQR: 22.1–27.7). The median interval between resection arthroplasty and reimplantation was 14.5 weeks (IQR: 11–23). Preoperative diagnoses before primary arthroplasty included 16 patients with osteoarthritis, 10 patients with femoral neck fractures, and 5 patients with osteonecrosis of the femoral head. Fourteen patients (14/31 = 45%, group A) suffered at least one spacer-related mechanical complication in the interim period. The other patients without mechanical complications (17/31 = 55%) comprised group B (Table 1). Six patients had spacer dislocation (19.4%), of which 4 were treated by closed reduction and immobilization with an abduction brace (Fig. 3); the 2 remaining patients underwent spacer exchange due to failure of closed reduction. Spacer fracture occurred in 3 patients (9.7%), 2 of whom were asymptomatic and were treated with conservative treatment. The remaining patient with a spacer fracture underwent spacer exchange due to recurrent infection. One patient who had a spacer dislocation and fracture underwent spacer exchange (3.2%). Femoral fractures occurred in 4 patients (12.9%); 2 suffered femoral fracture during resection and were treated with plating/wiring fixation, while the remaining 2 with femoral shaft fractures near the spacer stem were treated with conservative treatment.
Table 1.
Demographic data of patients in the spacer-complication group (group A) and the no-spacer-complication group (group B)
| Characteristics | Overall (n = 31) | Group A (n = 14) | Group B (n = 17) | p-value | |||
|---|---|---|---|---|---|---|---|
| Age, median (IQR), y | 56 | (50 to 71) | 55 | (41 to 59) | 64 | (52 to 77) | 0.021 |
| Male, n (%) | 19 | (61.3) | 10 | (71.4) | 9 | (52.9) | 0.290 |
| BMI, median (IQR), kg/m2 | 23.7 | (22.1 to 27.7) | 23.5 | (21.9 to 29.4) | 23.8 | (22.6 to 25.7) | 0.787 |
| ASA class, n (%) | 0.436 | ||||||
| 2 | 11 | (35.5) | 6 | (42.9) | 5 | (29.4) | |
| 3 | 20 | (64.5) | 8 | (57.1) | 12 | (70.6) | |
| Prosthesis type, n (%) | 0.895 | ||||||
| Bipolar | 8 | (25.8) | 3 | (21.4) | 5 | (29.4) | |
| Primary | 11 | (35.5) | 4 | (28.6) | 7 | (41.2) | |
| Revision | 12 | (38.7) | 7 | (50.0) | 5 | (29.4) | |
| Comorbidities, n (%) | |||||||
| Diabetes mellitus | 9 | (29.0) | 4 | (28.6) | 5 | (29.4) | 0.959 |
| Renal disease | 3 | (9.7) | 2 | (14.3) | 1 | (5.9) | 0.431 |
| Liver disease | 6 | (19.4) | 2 | (14.3) | 4 | (23.5) | 0.517 |
| Drug abuse | 3 | (9.7) | 2 | (14.3) | 1 | (5.9) | 0.431 |
| Alcoholism | 3 | (9.7) | 2 | (14.3) | 1 | (5.9) | 0.835 |
| Psychiatric disorder | 2 | (6.5) | 2 | (14.3) | 0 | (0) | 0.107 |
| Classification of PJI, n (%) | 0.003 | ||||||
| Acute | 10 | (32.3) | 1 | (7.1) | 9 | (52.9) | |
| Chronic | 21 | (67.7) | 13 | (92.9) | 8 | (47.1) | |
| 1st stage | |||||||
| Pre-op ESR, median (IQR), mm/hr | 63 | (30 to 91) | 62 | (28 to 96) | 63 | (30 to 76) | 0.889 |
| Pre-op CRP, median (IQR), mg/dL | 15 | (6.3 to 73.0) | 29 | (9.2–95) | 14.8 | (5.9 to 55.7) | 0.578 |
| Approach, n (%) | 0.031 | ||||||
| Posterior | 23 | (74.2) | 13 | (92.9) | 10 | (58.8) | |
| Direct lateral | 8 | (25.8) | 1 | (7.1) | 7 | (41.2) | |
| Paprosky bone defect classification, n (%) | |||||||
| Acetabulum | 0.143 | ||||||
| I | 14 | (45.2) | 5 | (35.7) | 9 | (52.9) | |
| II (A + B + C) | 8 | (25.8) | 6 | (42.9) | 2 | (11.8) | |
| III (A + B) | 9 | (29.0) | 3 | (21.4) | 6 | (5.3) | |
| Femur | 0.977 | ||||||
| I | 8 | (25.8) | 4 | (28.6) | 4 | (23.5) | |
| II | 10 | (32.3) | 4 | (28.6) | 6 | (35.3) | |
| III (A + B) | 11 | (35.5) | 5 | (35.7) | 6 | (35.3) | |
| IV | 2 | (6.4) | 1 | (7.1) | 1 | (5.9) | |
| Additional spacer exchange | 13 | (41.9) | 6 | (42.9) | 7 | (41.2) | 0.953 |
| Recurrent infection | 10 | 3 | 7 | ||||
| Interim perioda, median (IQR), weeks | 14.5 | (11 to 32) | 15 | (10 to 20) | 13.5 | (11 to 27) | 0.950 |
| Reimplantationa | |||||||
| Pre-op ESR, median (IQR), mm/hr | 29.9 | (11.5 to 47.0) | 31.2 | (14.5 to 46.0) | 28.8 | (8.3 to 55.5) | 0.397 |
| Pre-op CRP, median (IQR), mg/dL | 16.7 | (1.32 to 5.5) | 28.7 | (1.83 to 6.1) | 6.9 | (1.0 to 5.7) | 0.156 |
| Follow-up perioda, median (IQR), months | 29 | (23 to 35.5) | 29 | (25 to 34) | 30 | (23 to 36) | 0.948 |
aOne patient in group A and one patient in group B had permanent spacer implantation without revision surgery and were excluded from calculation of the interim period, CRP/ESR before reimplantation, and duration of follow-up period
IQR interquartile range, BMI body mass index, ASA American Society of Anesthesiologists, ESR erythrocyte sedimentation rate, CRP C-reactive protein, PJI periprosthetic joint infection
Fig. 3.
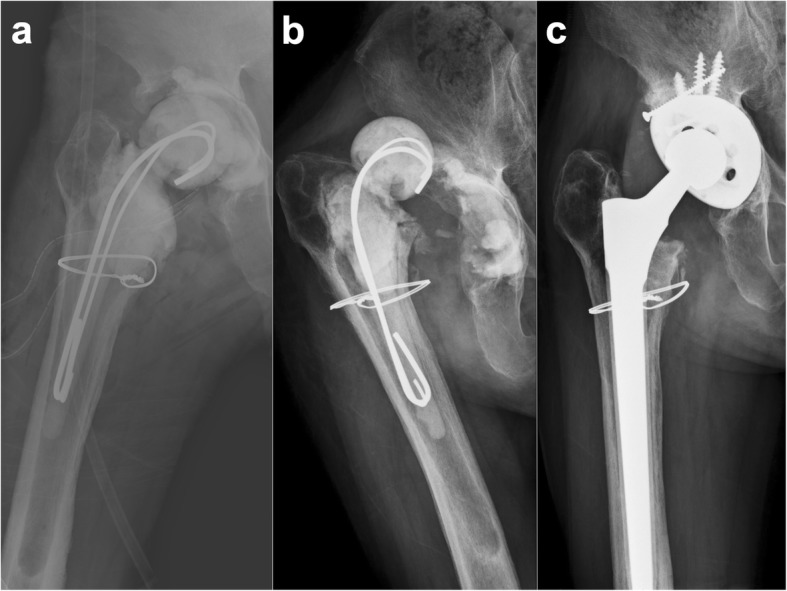
Hip radiographs showing: a) post-operative X-ray after implantation of a PMMA articulating spacer; b) dislocation of the PMMA articulating spacer 3 months later after implantation; and c) a well-fixed cementless total hip arthroplasty 2 years following reimplantation
The posterior approach was used in 92.9% of the patients in group A and 58.8% of the patients in group B (p = 0.031). Among patients in whom the posterior approach was employed, 7 suffered spacer dislocation. No patient experienced spacer dislocation when the direct lateral approach was used. Among the patients with spacer dislocation, according to the Paprosky classification, bone defects were classified as type I in 3 patients, type IIB in 2 patients, and type IIIA in 2 patients. There was no significant association between Paprosky type and spacer dislocation.
Thirteen patients underwent additional spacer exchange surgery in the interim period, including 6 patients in group A and 7 patients in group B. All 7 patients in group B who did not experience mechanical complications underwent additional spacer exchange surgery due to recurrent infection. Three of the 6 patients in group A had additional surgery for spacer exchange in the interim period owing to recurrent infection, one of whom had simultaneous spacer dislocation. The remaining 3 patients in group A underwent spacer exchange due to recurrent dislocation or fracture of the spacer. The rate of additional spacer exchange for mechanical complications or recurrent infection was not significantly different between the two groups (42.9% vs. 41.2%, p = 0.953).
In the whole cohort of 31 patients who were treated with articulating PMMA spacer implantation, gram-positive microorganisms caused the infection in the majority of cases. Coagulase-negative Staphylococcus was the most common pathogen (25.8%), followed by Staphylococcus aureus (19.4%) and Methicillin-resistant Staphylococcus aureus (6.5%). Culture-negative and polymicrobial PJI accounted for 22.6%and 16.1% of cases, respectively (Table 2).
Table 2.
Microbiologic laboratory data for specimens isolated during resection arthroplasty
| Pathogens, n (%) | ||
|---|---|---|
| Coagulase-negative Staphylococcus | 8 | (25.8) |
| Staphylococcus aureus | 6 | (19.4) |
| Methicillin-resistant Staphylococcus aureus | 2 | (6.5) |
| Streptococcus sanguinis | 1 | (3.2) |
| Pseudomonas aeruginosa | 1 | (3.2) |
| Escherichia coli | 1 | (3.2) |
| Polymicrobial | 5 | (16.1) |
| Culture-negative | 7 | (22.6) |
Patients who had mechanical complications were younger than those without mechanical complications (p=0.021). Chronic infection (p=0.003) and utilization of the posterior approach (p = 0.031) were risk factors for the development of spacer-related complications according to univariate analysis (Table 1). After adjusting for potential confounders (p < 0.10 in the univariate analysis), according to multivariate analysis, only a younger age (OR 0.91, 95% CI 0.83–1.00, p = 0.045) and chronic PJI (OR 14.7, 95% CI 1.19–182, p = 0.036) remained as risk factors associated with the development of spacer-related complications (Table 3).
Table 3.
Stepwise binary logistic regression between patients with spacer complications and those without
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
| Age (y) | 0.91 | 0.83–1.00 | 0.045 |
| Chronic infection | 14.7 | 1.19–182 | 0.036 |
CI confidence interval
One patient in each group retained the spacer without further reimplantation (Fig. 1). Twenty-nine patients eventually underwent reimplantation, and only one patient had a positive culture finding following reimplantation, revealing infection with Staphylococcus lugdunensis. That patient received intravenous and oral antibiotics for a total of 6 weeks, and no recurrent infection was noted during the follow-up period. One of the 29 patients died 3 months after reimplantation from a cause unrelated to PJI. Among the three patients with recurrent infection after reimplantation, two underwent resection arthroplasty, and the other underwent debridement with prosthesis retention. When stratified by spacer-related complications, the proportion of patients in whom treatment was successful after reimplantation was 84.6% (11/13) in the spacer-related-complications group (group A) versus 87.5% (14/16) in the non-spacer-complications group (group B). There was no statistically-significant difference between the two groups (log-rank test, p = 0.81) (Fig. 4).
Fig. 4.

Kaplan-Meier survival curve analysis of patients in whom treatment was successful in the spacer-related-complication group versus the no-spacer-complication group
In the 29 patients who underwent reimplantation, the treatment success rate following reimplantation was 86.2%(25/29). The median period of follow-up was 29 months (IQR: 23–35.5). The patients in group A had a lower median HHS (37; IQR: 30–42 vs. 60; IQR: 45.75–65.5, p<0.001) before reimplantation than those in group B, while the two groups had a similar median HHS after reimplantation (90; IQR: 78.5–92 vs. 89; IQR: 81.25–91.5, p=0.945) at the latest follow-up (Fig. 5).
Fig. 5.
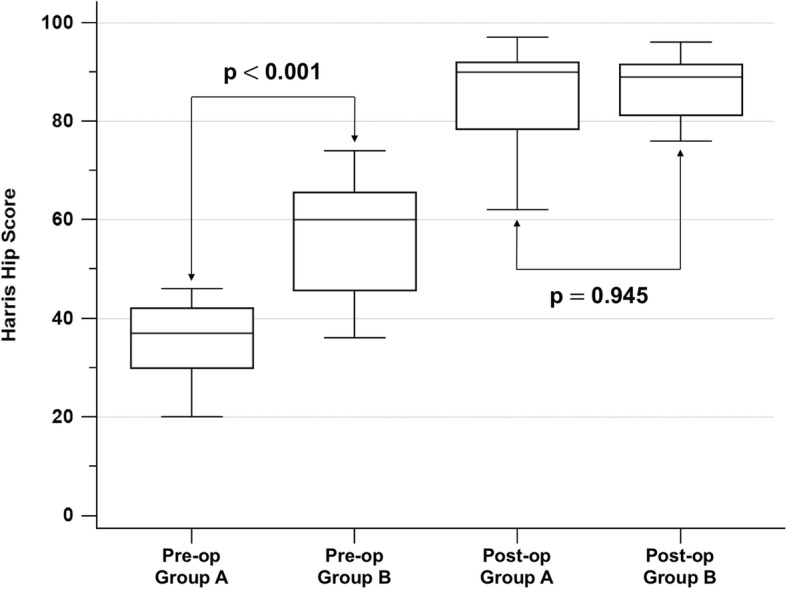
Box-and-whisker plots of preoperative and postoperative hip scores in the two groups
Discussion
Various rates of complications associated with articulating PMMA spacers have been reported in recent literature (Table 4), but no study has provided valuable evidence to substantiate the risk factors for such complications [13, 18, 19]. To our knowledge, this study was the first to demonstrate the risk factors associated with development of mechanical complications of articulating spacers in two-stage revision hip arthroplasty. Patients who experience articulating spacer complications have decreased functional activity in the interim period and do not benefit from the heightened mobility that articulating spacers can provide. Although mechanical complications might require exchange of the articulating spacer in the interim period, most of our patients (78.6%, 11/14) did not undergo additional spacer exchange surgery, instead having a shortened interim period before reimplantation. Therefore, surgeons should aim to prevent the occurrence of mechanical complications of articulating spacers in order to maintain quality of life in the interim period for patients undergoing two-stage exchange arthroplasty.
Table 4.
Literature regarding hip-spacer complications
| Study | No. of patients | Spacer type | Spacer mechanical complications | Overall | Outcome after reimplantation |
|---|---|---|---|---|---|
| Duncan et al. (1993) [12] | 13 | PROSTALAC | 3 dislocations | 23% |
1 allograft nonunion (8%) 1 loose Lord ring (8%) 2 heterotopic bones (15%) |
| Ivarsson et al. (1994) [26] | 5 | Hand-made |
1 dislocation 1 subtrochanteric fracture |
40% | – |
| Leunig et al. (1998) [18] | 12 | Hand-made |
5 dislocations 1 spacer fracture |
50% | – |
| Younger et al.(1998) [3] | 29 (30 spacers) | PROSTALAC | 3 dislocations | 10% |
1 extrusion of cement into knee (3%) 1 recurrent infection (3%) 2 recurrent dislocations (7%) 2 allograft nonunions (7%) 1 loose Lord ring (3%) 3 prosthesis migrations (10%) |
| Magnan et al. (2001) [27] | 10 | Mold | 1 dislocation | 10% | – |
| Koo et al. (2001) [28] | 22 | Mold | 1 femur iatrogenic fracture | 5% |
1 recurrent infection (5%) 2 peroneal nerve palsies (9%) 1 greater trochanteric non-union (5%) 3 heterotopic ossifications (14%) |
| Durbhakula et al. (2004) [14] | 20 | Mold |
2 dislocations 2 spacer fractures |
20% | – |
| Hsieh et al. (2004) [4] | 58 | Mold |
2 dislocations 2 spacer fractures |
7% |
2 recurrent infections (3%) 1 dislocation (2%) |
| Jung et al. (2009) [13] | 82 (88 spacers) | Mold |
15 spacer dislocations 9 spacer fractures 12 femoral fractures |
40.9% | 16 dislocations (23%) |
| Faschingbauer et al. (2015) [15] | 138 |
93 mold 45 hand-made |
12 dislocations 12 spacer fractures 1 femoral fracture 1 spacer fracture-dislocation 1 spacer protrusion into pelvis |
19.5% | – |
| This study | 35 | Mold |
6 dislocations 3 spacer fractures 1 spacer fracture-dislocation 4 femoral fractures |
40% |
3 recurrent infections 1 dislocation 1 greater trochanteric non-union |
A primary finding of our study was that younger patients were much more likely to suffer mechanical complications than older patients. The high demand placed on the spacer during daily activity in younger patients may significantly predispose them to excess stress, leading to mechanical complications (i.e., spacer fractures). In our study, the metallic endoskeletons were composed of three large Kirschner wires. Such types of metallic endoskeleton may predispose spacers to fractures [18]. Hsieh et al. reported two fractures of the cement femoral stem in 24 patients (8.3%), which was similar to our study (9.7%) [20]. Given that younger patients have higher functional demands in daily activity, stronger metallic endoskeletons, such as preformed spacers with reinforced metal cores [12, 21], should be considered to provide mechanical integrity and minimize the risk of spacer fracture.
Chronic PJI was also identified as a primary risk factor for mechanical complications in the patients with articulating spacers. Individuals with chronic infections locally are subject to deleterious bone destruction from chronic inflammation, which may propagate defects detrimental to spacer fixation at the joint [3, 20, 22]. As there was no significant difference in Paprosky classification between groups A and B in our study, we believe that chronic PJI of the hip may result in poor bone quality, leading to mechanical complications of articulating spacers. Additionally, although all study patients underwent the same standard rehabilitation program following spacer implantation, iatrogenic femur fractures only occurred in the interim period in patients with chronic PJI.
In our study, we observed spacer-related complications in the majority (92.9%) of patients who underwent the procedure via the posterior approach. Recent literature has described the posterior approach as resulting in a higher dislocation rate in comparison to the direct lateral approach in THA [23, 24]; however, with meticulous and secure closure of the posterior capsule, the dislocation rate can be lowered to a rate comparable to that seen in patients in which the direct lateral approach is used [25]. Patients with PJI require extensive debridement, and this may present a problem with regards to adequate closure of the posterior capsule, which may lead to instability. Therefore, we hypothesize that the dislocation rate following spacer implantation after PJI may increase with use of the posterior approach.
The success rate for infection eradication under the two-stage revision protocol is around 82 to 96% [3–8], and our result (86.2%) was consistent with previous literature. Furthermore, the clinical outcome was not different in the two study groups following reimplantation.
There were several limitations to our study that we would like to acknowledge. The first was regarding the retrospective nature of the study, in addition to the small study cohort. The cohort size resulted from our institution’s patient cohort treated with articulating spacer implantation. Second, we did not stratify each category of spacer complication owing to the small cohort size. Finally, we were unable to measure the precise amount of time following which each patient could bear weight on the implant, which may have contributed to the occurrence of spacer-related complications.
Conclusion
Our study demonstrated a high complication rate for articulating PMMA spacers in two-stage revision hip arthroplasty. We identified a young age and chronic PJI as the primary risk factors for development of mechanical spacer complications. Surgeons should remain cautious when using articulating spacers in patients with cranial defects of the acetabulum and those who have reduced containment of the spacer within the hip joint. Further research is needed in order to eliminate spacer-associated complications in order to provide a better patient experience during the interim stage.
Acknowledgements
We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for their supporting statistical work.
Abbreviations
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- CI
Confidence interval
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- Hb
Hemoglobin
- HHS
Harris hip score
- IQR
Interquartile range
- OD
Odds ratio
- PJI
Periprosthetic joint infection
- PMMA
Polymethylmethacrylate;
- SD
Standard deviation
- THA
Total hip arthroplasty
Authors’ contributions
FSY and FCK were involved in the conception and design of the study and writing of the manuscript. FSY, MSL and CTW collected and analyzed the data. YDL and KB were involved in the literature search and editing of the manuscript. FCK and MSL supervised the version to be published. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data are available from the corresponding author.
Ethics approval and consent to participate
The protocols used in this retrospective study were reviewed and approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 201701676B0). According to Taiwanese national legislation, patient consent is not required in retrospective studies.
Consent for publication
Not applicable.
Competing interests
All authors declared that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fu-Shine Yang, Email: bb1220@cgmh.org.tw.
Yu-Der Lu, Email: rexru@cgmh.org.tw.
Cheng-Ta Wu, Email: oliverwu429@cgmh.org.tw.
Kier Blevins, Email: kier.blevins@duke.edu.
Mel S. Lee, Email: mellee@cgmh.org.tw
Feng-Chih Kuo, Phone: +886-7-7317123, Email: fongchikuo@cgmh.org.tw.
References
- 1.Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. J Bone Joint Surg Am. 2015;97(18):1495–1502. doi: 10.2106/JBJS.N.00958. [DOI] [PubMed] [Google Scholar]
- 2.Tikhilov R, Bozhkova S, Denisov A, Labutin D, Shubnyakov I, Razorenov V, Artyukh V, Klitsenko O. Risk factors and a prognostic model of hip periprosthetic infection recurrence after surgical treatment using articulating and non-articulating spacers. Int Orthop. 2016;40(7):1381–1387. doi: 10.1007/s00264-015-3072-4. [DOI] [PubMed] [Google Scholar]
- 3.Younger AS, Duncan CP, Masri BA. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J Bone Joint Surg Am. 1998;80(1):60–69. doi: 10.2106/00004623-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh PH, Shih CH, Chang YH, Lee MS, Shih HN, Yang WE. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;86-A(9):1989–1997. doi: 10.2106/00004623-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89(4):871–882. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh RG, Thevarajan K, Kok CS, Sivapathasundaram N, George SV. An intramedullary cement spacer in total hip arthroplasty. J Arthroplast. 1998;13(2):197–199. doi: 10.1016/S0883-5403(98)90099-7. [DOI] [PubMed] [Google Scholar]
- 7.Marczak D, Synder M, Sibinski M, Polguj M, Dudka J, Kowalczewski J. Two stage revision hip arthroplasty in periprosthetic joint infection. Comparison study: with or without the use of a spacer. Int Orthop. 2017;41(11):2253–2258. doi: 10.1007/s00264-017-3500-8. [DOI] [PubMed] [Google Scholar]
- 8.Vielgut I, Sadoghi P, Wolf M, Holzer L, Leithner A, Schwantzer G, Poolman R, Frankl B, Glehr M. Two-stage revision of prosthetic hip joint infections using antibiotic-loaded cement spacers: when is the best time to perform the second stage? Int Orthop. 2015;39(9):1731–1736. doi: 10.1007/s00264-015-2751-5. [DOI] [PubMed] [Google Scholar]
- 9.Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. J Bone Joint Surg Am. 1994;76:1742–1751. doi: 10.2106/00004623-199411000-00020. [DOI] [Google Scholar]
- 10.Choi HR, Freiberg AA, Malchau H, Rubash HE, Kwon YM. The fate of unplanned retention of prosthetic articulating spacers for infected total hip and total knee arthroplasty. J Arthroplast. 2014;29(4):690–693. doi: 10.1016/j.arth.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Petis SM, Perry KI, Pagnano MW, Berry DJ, Hanssen AD, Abdel MP. Retained antibiotic spacers after Total hip and knee arthroplasty resections: high complication rates. J Arthroplast. 2017;32(11):3510–3518. doi: 10.1016/j.arth.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Duncan CP, Beauchamp C. A temporary antibiotic-loaded joint replacement system for management of complex infections involving the hip. Orthop Clin North Am. 1993;24(4):751–759. [PubMed] [Google Scholar]
- 13.Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci. 2009;6(5):265–273. doi: 10.7150/ijms.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Spacer Endoprosthesis for the treatment of infected Total hip arthroplasty. J Arthroplast. 2004;19(6):760–767. doi: 10.1016/j.arth.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Faschingbauer M, Reichel H, Bieger R, Kappe T. Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty. Int Orthop. 2015;39(5):989–994. doi: 10.1007/s00264-014-2636-z. [DOI] [PubMed] [Google Scholar]
- 16.Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J. 2015;97-b(Suppl A):20–29. doi: 10.1302/0301-620X.97B10.36475. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of Periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471(7):2374–2382. doi: 10.1007/s11999-013-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leunig M, Chosa E, Speck M, Ganz R. A cement spacer for two-stage revision of infected implants of the hip joint. Int Orthop. 1998;22(4):209–214. doi: 10.1007/s002640050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthop. 2006;77(4):628–637. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh PH, Shih CH, Chang YH, Lee MS, Yang WE, Shih HN. Treatment of deep infection of the hip associated with massive bone loss. J Bone Joint Surg (Br) 2005;87(6):770–775. doi: 10.1302/0301-620X.87B6.15411. [DOI] [PubMed] [Google Scholar]
- 21.Younger ASE, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplast. 1997;12(6):615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]
- 22.Charlton WP, Hozack WJ, Teloken MA, Rao R, Bissett GA. Complications associated with reimplantation after Girdlestone arthroplasty. Clin Orthop Relat Res. 2003;407:119–126. doi: 10.1097/00003086-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary Total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456–2463. doi: 10.2106/JBJS.D.02860. [DOI] [PubMed] [Google Scholar]
- 24.Svenøy S, Westberg M, Figved W, Valland H, Brun OC, Wangen H, Madsen JE, Frihagen F. Posterior versus lateral approach for hemiarthroplasty after femoral neck fracture: early complications in a prospective cohort of 583 patients. Injury. 2017;48(7):1565–1569. doi: 10.1016/j.injury.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Parker MJ. Lateral versus posterior approach for insertion of hemiarthroplasties for hip fractures: a randomised trial of 216 patients. Injury. 2015;46(6):1023–1027. doi: 10.1016/j.injury.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Ivarsson I, Wahlström O, Djerf K, Jacobsson S-A. Revision of infected hip replacement: two-stage procedure with a temporary gentamicin spacer. Acta Orthop Scand. 1994;65(1):7–8. doi: 10.3109/17453679408993707. [DOI] [PubMed] [Google Scholar]
- 27.Magnan B, Regis D, Biscaglia R, Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics. Acta Orthop Scand. 2001;72(6):591–594. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 28.Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kim SY, Kim YH. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplast. 2001;16(7):882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author.



