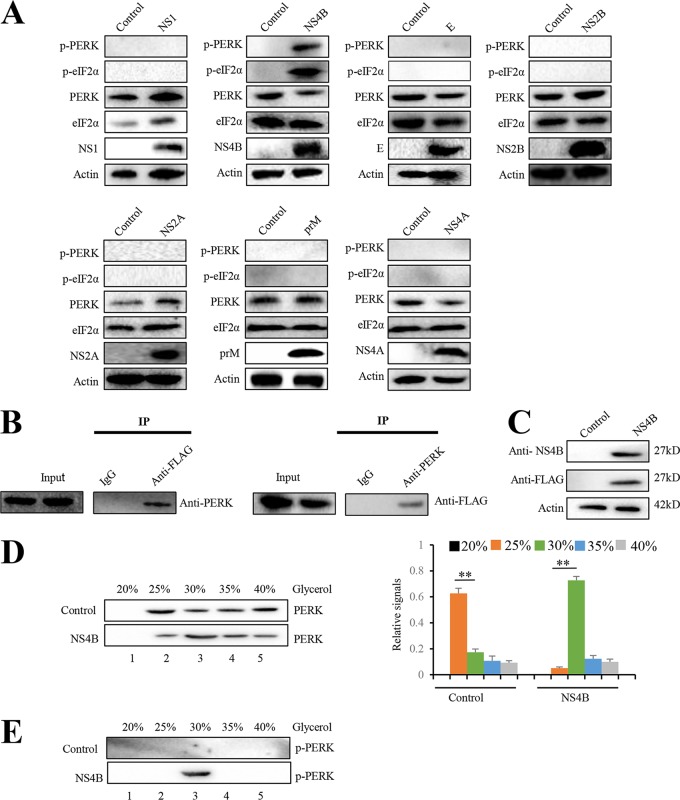
FIG 4.
JEV NS4B activates PERK by promoting its dimerization. (A) Neuro-2a cells were transfected with plasmid expressing prM, E, NS1, NS2A, NS2B, NS4A, or NS4B. Empty vector-transfected Neuro-2a cells were used as negative controls. After 2 days, the cells were subjected to immunoblot analysis using PERK, eIF2α, phospho-PERK, phospho-eIF2α, FLAG, or actin antibody. Representative images from three independent experiments are shown. (B) Neuro-2a cells were transfected with the plasmid expressing NS4B-3FLAG. After 2 days, the cell lysates were immunoprecipitated with anti-FLAG antibody (left) or anti-PERK antibody (right). The resulting immunoprecipitates (IP) and whole-cell lysates used for immunoprecipitation (Input) were examined by an immunoblot assay with anti-FLAG antibody for detecting NS4B and anti-PERK antibody. The negative-control sample was generated by using control IgG antibody. Representative images from three independent experiments are shown. (C to E) Neuro-2a cells were transfected with plasmid expressing NS4B or empty vector. After 2 days, cellular lysates were subjected to immunoblot analysis with NS4B, FLAG, or actin antibody (C). For the analysis of PERK dimerization, cellular lysates were separated using a 20 to 40% glycerol gradient. PERK (D) and phosphorylated PERK (E) in gradient fractions were determined by immunoblotting. Fractions are numbered 1 to 5 in order from top to bottom (light to heavy), respectively. The left panel (D) shows representative images; the right panel (D) shows quantitation data. Error bars indicate the SD of the mean (n = 3). **, P < 0.01.