Abstract
Objectives
The objective of this study was to evaluate the therapeutic effects of balanitoside in diabetic rats.
Methods
Twenty-five rats were divided into five groups. Rats in groups 2 to 5 were treated with streptozotocin to induce hyperglycemia. In addition, rats in groups 1 and 2 received 1 mL of distilled water, whereas those in groups 3, 4, and 5 received 10 and 20 mg/kg balanitoside and 6 U/kg insulin, respectively, for 14 days. All rats were sacrificed on day 15, blood samples were collected, and serum levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured. The liver was processed for examination under a light microscope.
Results
The results showed a significant decrease in liver protein concentrations in diabetic control rats, compared to those in the normal control rats and rats treated with 10 mg/kg balanitoside (p < 0.05). There was no significant difference in ALP levels among all groups. However, a significant increase in ALT and AST levels was observed in the diabetic control rats, compared to those in the normal control rats (p < 0.05). Photomicrographs of the liver of the diabetic control rats showed fat and glycogen droplets, vacuolated nuclei, and loss of cellular boundaries, whereas those of the rats treated with balanitoside or insulin showed a small amount of microvesicular fat droplets and slight infiltration of lymphocytes.
Conclusion
The findings of this study suggest the therapeutic effects of balanitoside in the liver of diabetic rats.
Keywords: Balanitoside, Diabetes mellitus, Hyperglycemia, Liver, Protein concentration
Introduction
Diabetes mellitus is characterized by hyperglycemia owing to either a decrease in insulin production, insulin resistance, or both.1 It can be classified into type 1, which results from autoimmune destruction of the pancreatic beta cells, and type 2, which occurs owing to insulin resistance or deficiency.2 The symptoms of diabetes mellitus include polyuria, polydipsia, and polyphagia. The liver plays an important role in carbohydrate homeostasis, where accumulation of glycogen in the liver induces liver enzyme abnormalities in uncontrolled diabetic patients.2 Non-alcoholic fatty liver disease (NAFLD) is a common complication of diabetes, usually observed in patients with type 2 diabetes and can progress to liver cirrhosis. The prevalence of NAFLD in the general population ranges between 20 and 30%; however, its prevalence is approximately 75% in diabetic patients. In addition, the mortality rate of liver cirrhosis in diabetic patients is more than twice of that in the general population.3 Three different hypotheses can explain the relationship between diabetes and liver disease: liver disease might induce diabetes, diabetes might contribute to or induce liver disease, or the risk factors for liver disease and diabetes are similar.4
Balanitoside is the active pharmacological ingredient of Balanites aegyptiaca. It is a saponin that belongs to a family of structurally related compounds of steroids or triterpenoid aglycones linked to one or more oligosaccharide moieties by glycoside linkages; the carbohydrate moiety consists of pentoses, hexoses, or uronic acids. The presence of both polar (sugar) and nonpolar steroid groups provides saponins with strong surface active properties, which distinguish them from other glycosides.5 Saponins exhibit various pharmacological activities, including expectorant, anti-inflammatory, vasoprotective, antioxidant, hypocholesterolemic, immunomodulatory, hypoglycemic, molluscicidal, antifungal, and antiparasitic activities.6 In this study, we aimed to investigate the therapeutic effects of balanitoside in diabetic rats.
Materials and Methods
Balanitoside was isolated from B. aegyptiaca fruits, as previously described.7 Twenty-five Wistar rats (150–200 g) were obtained from the animal house, Department of Pharmacology and Therapeutics, ABU, Zaria, Nigeria. They were fed standard food pellets (Vital Feed, Nigeria) and provided water ad libitum. They were allowed to acclimatize to the conditions in the animal house for 2 weeks.
Hyperglycemia was induced using streptozotocin, which was freshly prepared in 0.1 M citrate buffer (pH 4.5), as previously described.8 Rats were divided into five groups (n = 5 each). Rats in groups 1 and 2 served as positive and negative control groups, respectively, and received 1 mL of distilled water for 14 days. Rats in groups 3, 4, and 5 intraperitoneally received 10 and 20 mg/kg balanitoside and 6 U/kg insulin, respectively, for 14 days. All rats were killed on day 15, and blood samples were collected in plain bottles via cardiac puncture. The liver was harvested, processed, stained with hematoxylin & eosin (H & E) and periodic acid-Schiff (PAS), and examined under a light microscope.
The blood samples were centrifuged at 2500 ×g for 5 min, and the serum was collected. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were determined using enzyme-linked immunosorbent assay (ELISA) kits.
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software, version 20 (IBM, Armonk, New York, USA). Data are expressed as the means ± standard deviations (SD). One-way analysis of variance (ANOVA) was used to compare the differences among groups (turkey test). p < 0.05 was considered statistically significant.
Results
There was no significant difference in ALP levels among all groups (p = 0.138). There was a significant increase in the level of ALT between the normal control rats and the diabetic control rats and rats treated with Balanitoside and insulin (p = 0.001). There was a significant increase in the level of AST between the normal control rats and the diabetic control rats and rats treated with Balanitoside and insulin (p = 0.023). Liver protein concentrations in diabetic control rats was significantly lower than those in normal control rats and rats treated with 10 mg/kg balanitoside (p = 0.01). There was no significant difference in protein concentrations between the diabetic control rats and rats treated with 20 mg/kg balanitoside or 6 U/kg insulin (see Table 1).
Table 1.
Effects of balanitoside on liver enzymes and plasma protein concentrations in streptozotocin-induced diabetic rats.
| Parameters | Normal control | Diabetic control | 10 mg/kg Balanitoside |
20 mg/kg Balanitoside |
6 U/kg Insulin | P-value |
|---|---|---|---|---|---|---|
| ALP (U/L) | 65 ±7a | 72.2 ± 3.83a | 68.2 ± 10.42a | 75.4 ± 6.77a | 67.2 ± 1.3a | 0.138 |
| ALT (U/L) | 40.44 ± 4.11a | 47.2 ± 2.38b | 47.8 ± 3.34b | 56.2 ± 2.77c | 48.4 ± 4.27b | 0.001 |
| AST (U/L) | 35.6 ± 1.14a | 43 ± 3.8b | 42.4 ± 2.07ab | 48.2 ± 8.25b | 44.8 ± 3.7b | 0.023 |
| PPC (mg/dL) | 107.98 ± 33.43b | 79.22 ± 11.17a | 105.98 ± 4.56b | 92.05 ± 5.98ab | 100.5 ± 8.39ab | 0.01 |
Values expressed as Mean ± SD, values in the same row with different superscripts are significantly different at (p ≤ 0.05). STZ – Streptozotocin, ALP – alkaline phosphatase, ALT – alanine transaminase, AST – aspartate transaminase, PPC – plasma protein concentration.
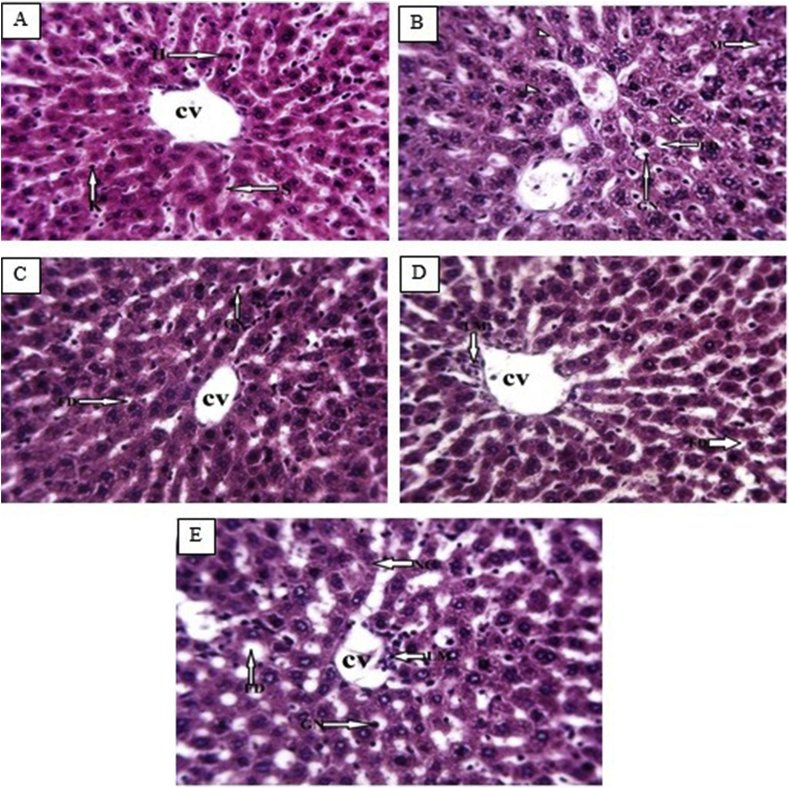
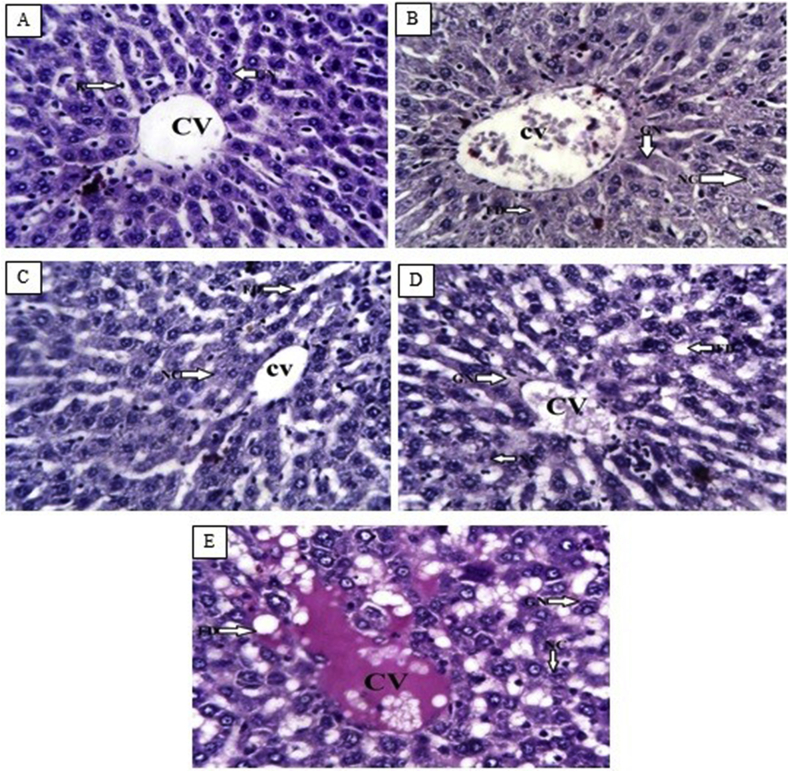
Photomicrographs of the liver of the normal control rats showed normal hepatocytes, sinusoids, and central vein (Figure 1, Figure 2A). However, the diabetic control rats showed fatty droplets, vacuolated nuclei, cellular necrosis, glycogen deposits in the central vein and hepatocytes, and hemorrhage (Figure 1, Figure 2B). Rats treated with 10 mg/kg balanitoside showed normal central vein and hepatocytes with a small amount of microvesicular fat droplets (Figure 1, Figure 2C). Rats treated with 20 mg/kg balanitoside showed vacuolated nuclei, macrovesicular fat droplets, and infiltration of lymphocytes around the central vein (Figure 1, Figure 2D), whereas those treated with 6 U/kg insulin showed slight infiltration of lymphocytes within the central vein, degenerated sinusoids, vacuolated hepatocytes, and a small amount of microvesicular fat droplets (Figure 1, Figure 2E).
Figure 1.
Photomicrographs of liver tissues from normal control rats, diabetic control rats, and rats treated with 10 and 20 mg/kg balanitoside and 6 U/kg insulin showing hepatocytes (H), fatty droplets (FD), sinusoid (S), giant mitochondria (GN), necrotic cells (NC), lymphocytes (LM), and central vein (CV) (H & E stain; ×250).
Figure 2.
Photomicrographs of liver tissues from normal control rats, diabetic control rats, and rats treated with 10 and 20 mg/kg balanitoside and 6 U/kg insulin showing hepatocytes (H), sinusoid (S), fatty droplets (FD), vacuolated nuclei (GN), necrotic cells (NC), and central vein (CV) (PAS stain; ×250).
This study was limited to measurement of the serum levels of ALP, ALT, and AST, as well as examination of the liver structure using H & E and PAS under a light microscope in diabetic rats.
Discussion
The decrease in liver protein concentrations in the diabetic control rats is in line with the findings of previous studies.14 It might be attributed to the increase in the rate of protein catabolism, decrease in the rate of protein synthesis, or microproteinuria, which are important clinical markers of diabetes mellitus. Increased insulin levels usually result in increased protein synthesis, thereby reducing protein metabolism.9 The protein concentrations in rats treated with balanitoside or insulin were within the normal range (90–110 mg/dL), according to Hoffman's method.10
The significant increase in the levels of ALT and AST in the diabetic control rats suggested that diabetes mellitus could induce hepatic injury, which might be owing to the increase in protein concentrations accompanying glucogenesis and urea formation observed in the diabetic state. ALT and AST are directly associated with the conversion of amino acids to keto acids and are reported to increase in diabetic conditions.11 Rats treated with 10 mg/kg balanitoside or insulin showed a mild increase in ALT and AST levels, compared to the rats treated with 20 mg/kg balanitoside, indicating that balanitoside at 10 mg/kg might exhibit therapeutic effects in the hepatocytes by reducing the levels of ALT and AST. However, elevation of ALT and AST levels in the rats treated with insulin could be attributed to impairment in insulin signaling rather than hepatic injury because ALT and AST gene transcription is suppressed by insulin.12 The insignificant increase in ALP levels in the rats treated with 10 mg/kg balanitoside or insulin, compared to those in the normal control rats, suggested that balanitoside at 10 mg/kg might exhibit therapeutic effects in the hepatocytes. The presence of fat and glycogen droplets, vacuolated nuclei, and loss of cellular boundaries observed in the liver of the diabetic control rats indicated that diabetes mellitus co-existed with NAFLD, which is in line with the findings of a previous study,13 which showed that hepatic accumulation of fat and glycogen are common complications of diabetes mellitus reported in 80% of patients with diabetes. Glycogen accumulates in patients with diabetes because of insulin deficiency that may increase synthase activity resulting in increased rate of glucogenesis and more glycogen accumulation.14 Type 1 diabetes is not associated with fat accumulation if blood glucose level is well-controlled; however, fat accumulation occurs in 70% of patients with type 2 diabetes regardless of blood glucose control.15 The improvement in liver histology in the rats treated with balanitoside or insulin indicated that balanitoside might exhibit therapeutic effects in the hepatocytes.
Conclusion
Balanitoside at 10 mg/kg exerted therapeutic and antioxidant effects in the liver of streptozotocin-induced diabetic rats by the following ways:
-
1.
Decreasing the plasma levels of oxidative stress markers (ALP, ALT, and AST) and correcting streptozotocin-induced hypoproteinemia, and
-
2.
Increasing the rate of regeneration and healing of the damaged liver tissues.
Recommendation
More studies are needed to investigate the effects of balanitoside on the plasma levels of other oxidative stress markers, such as superoxide dismutase (SOD), catalase (CAT), and malondialdehyde, as well as on the ultrastructure of the liver in diabetic rats.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The research was conducted in accordance with ABU, Zaria Research and Ethical Committee and the ARRIVE Guidelines (Reporting of in Vivo Experiment).
Authors' contributions
WM, WOH, AAB, and NID conceived and designed the study. WM and SGO provided research materials. WOH and AAB provided administrative support. WM, NID, and SGO conducted the research and collected and organized the data. WM, WOH, AAB, and NID analyzed and interpreted the data. WM and NID wrote the initial and final draft of the manuscript and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Cunha A.P., Ribeiro A.C.B., Ricardo N.M.P.S., Oliveira A.C., Davila L.S.P., Cardoso J.H.L. Polysaccharides from Caesalpinia ferrea seeds-chemical characterization and anti-diabetic effects in Wistar rats. Food Hydrocoll. 2017;65:68–76. [Google Scholar]
- 2.Acharya A., Wudneh E., Krishnan R., Ashraf A., Tohid H. Diabetes and liver an association: hepatogenous diabetes mechanism and some evidences. J Cell Sci Ther. 2016;7:257. [Google Scholar]
- 3.Jia G., Di F., Wang Q., Shao J., Gao L., Wang L. Non-alcoholic fatty liver disease is a risk factor for the development of diabetic nephropathy in patients with type 2 diabetes mellitus. PLoS One. 2015;10(11):1–11. doi: 10.1371/journal.pone.0142808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H. Epidemic obesity and type II diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 5.Yu F.C., Chao H.Y., Ming S.C., Yong-Ping C., Yu-Chun H. Foam properties and detergent abilities of the Saponins from Camellia oleifera. Int J Mol Sci. 2010;11:4417–4425. doi: 10.3390/ijms11114417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahu N.P., Banerjee S., Mondal N.B., Mandal D. Springer; Vienna: 2008. Steroidal saponins in Fortschritte der Chemie Organischer Naturstoffe progress in the chemistry of organic natural products. [DOI] [PubMed] [Google Scholar]
- 7.Wiart C., Hannah A., Yassim M., Hamimah H., Sulaiman M. Antimicrobial activity of Acalypha siamensis Oliv. ex Gage. J Ethnopharmacol. 2004;95:285–286. doi: 10.1016/j.jep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Al-Attar A.M., Zari T.A. Modulatory effects of ginger and clove oils on physiological responses in streptozotocin-induced diabetic rats. Int J Pharmacol. 2007;3:34–40. [Google Scholar]
- 9.El-Sayed M.I., Awad S., Wahba A., El Attar A., Yousef M.I., Zedan M. In Vivo anti-diabetic and biological activities of milk protein and milk protein hydrolyaste. Adv Dairy Res. 2016;4:154. doi: 10.3168/jds.2015-10626. [DOI] [PubMed] [Google Scholar]
- 10.Murugan P., Pari L. Influence of tetrahydrocurcumin on hepatic and renal functional markers and protein levels in experimental type 2 diabetic rats. Basic Clin Pharmacol Toxicol. 2007;101:241–245. doi: 10.1111/j.1742-7843.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T. Central Clinical Laboratory and Research Division Nihon University Surugadai Hospital; Tokyo: 1974. Clinical aspects of the plasma proteins. [Google Scholar]
- 12.Begum N., Shanmugasundaram K.R. Transaminases in experimental diabetes. Arogya J Health Sci. 1978;4:116–122. [Google Scholar]
- 13.O'Brien R.M., Granner D.K. Regulation of gene expression by insulin. Biochem J. 1991;278:609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suriawinata A.A., Thung S.N. 2011. Liver pathology an Atlas and concise guide. Latest Edition 2011. [Google Scholar]
- 15.Silverman J.F., O'Brien K.F., Long S., Leggett N., Khazanie P.G., Pories W.J. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. [PubMed] [Google Scholar]