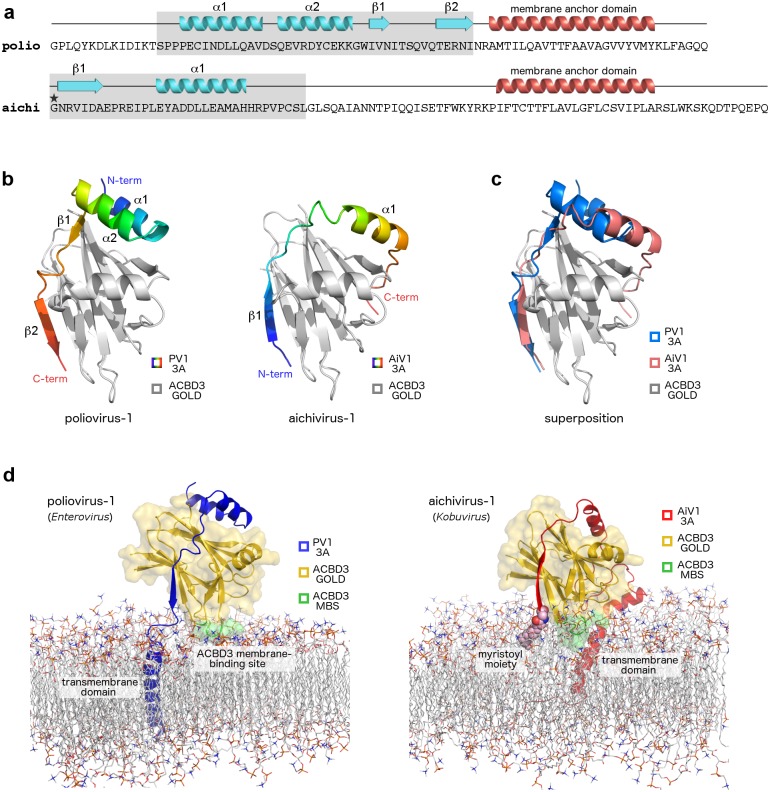
Fig 7. Convergence in the mechanisms of ACBD3 recruitment by enteroviruses and kobuviruses.
a, Distinct ACBD3-binding regions of enterovirus and kobuvirus 3A proteins. Sequences of the poliovirus-1 (member of enteroviruses) and aichivirus-1 (member of kobuviruses) 3A proteins are shown. Secondary structures present in the crystal structures of the ACBD3: 3A complexes (colored in light blue) and the hydrophobic alpha helices anchoring the 3A proteins to the membrane (colored in red) are indicated above the sequences. ACBD3-binding regions are shaded in grey. Myristoylated Gly1 of the aichivirus-1 3A protein is marked with an asterisk. b, Crystal structures of the ACBD3 GOLD domain in complex with the poliovirus (left) and aichivirus (right) 3A proteins. The protein backbones are shown in cartoon representation. The ACBD3 GOLD domain is depicted in grey, the viral 3A proteins in rainbow colors from blue (N terminus) to red (C terminus). c, Superposition of the crystal structures from (b). The ACBD3 GOLD domain is depicted in grey, the poliovirus 3A protein in blue, the aichivirus 3A protein in red. d, Molecular dynamics simulation-based models of the ACBD3 GOLD domain in complex with the poliovirus (left) and aichivirus (right) 3A proteins on the lipid bilayer. The ACBD3 GOLD domain is shown in cartoon representation with a semi-transparent surface and colored in gold except for the membrane binding site, which is colored in green. The poliovirus 3A protein is depicted in blue, the aichivirus 3A protein in red.