Abstract
Risk for developing mental health concerns is increased for people with diabetes. Coupled with stressors related to the transition from adolescence to adulthood, emergent adults may be in greater need of psychosocial interventions to help them cope. This review summarizes the literature on interventions used with people with diabetes aged 15–30 years on psychosocial and biological (A1C) outcomes. Core databases were searched for both published and grey research. Studies completed between January 1985 and October 2018 using any psychosocial intervention and meeting age and diabetes type requirements were selected if they included a control or comparison group and findings reported in such a way that effect size was calculable. Two authors independently extracted relevant data using standard data extraction templates. Six studies with 450 participants met the broad inclusion criteria. Sample-weighted pooling of 12 outcomes, six each on glycemic control and psychosocial status, suggested the preventive potential (d = 0.31, 95% CI 0.17–0.45) and homogeneity (χ2 [11] = 11.15, P = 0.43) of studied interventions. This preliminary meta-analysis provides some suggestion that psychosocial interventions, including telephone-based case management, individualized treatment modules, and small-group counseling interventions, may diminish burden, depression, and anxiety and enhance glycemic control among emerging adults with type 1 diabetes as they transition from adolescence to adulthood.
Developmental psychologist Jeffrey Arnett describes emergent adulthood as an exploratory and sometimes unstable phase of life when one is no longer an adolescent, but not yet an adult (1). Emergent adults contend with a variety of challenges and transitions, such as to independent living, post-secondary education, full-time employment, and increasingly intimate relationships, perhaps including marriage and parenthood. These transitions are not always simple; consequently, emergent adults seem to be at increased risk for many physical and mental health challenges. For example, the highest incidence rates of depression and suicide are found among young men and women aged 15–24 years in both Canada and the United States (2,3). Therefore, it seems reasonable to assume that the addition of a chronic illness such as type 1 diabetes to this transitional stage greatly increases the chances of burdensome challenges (4).
Longitudinal primary and synthetic research in this field suggests that emergent adults with type 1 diabetes are more likely to have mental health concerns than are their peers without diabetes. Several studies have confidently estimated that there are increased chances of emergent adults with type 1 diabetes developing depression, distress, or low life satisfaction over time (5–12). Most troubling in terms of longer-term socioeconomic risks have been findings that emergent adults with type 1 diabetes are significantly less likely to complete their educations and twice as likely to be diagnosed with major depression than are their age-matched peers without diabetes (10,11). Related studies have consistently found similarly grave differences on suicidal ideation and suicide. In fact, mortality rate ratios among emergent adults with or without type 1 diabetes range from 2.00 to 5.00, with most of the excess deaths among such young people with diabetes accounted for by suicides and accidents (13,14). Therefore, the importance of providing them with the highest-quality health care, including mental health care, is clear, and the preventive potential of doing so is equally clear.
Because diabetes regimen compliance and self-management are probably negatively associated with socioeconomic status (SES) and lower SES disproportionately affects members of racial and ethnic minority groups, these groups should be included in this field’s research syntheses (15–19). Both primary and synthetic research has suggested that indigenous people in Canada and African-American and Hispanic people in the United States with type 1 diabetes are at much greater risk of both poor glycemic control and mental health problems, socioeconomic factors presenting a likely explanation for their apparent barriers (15–19). Our understandings here are undeveloped, as noted in a previous systematic review that such sociodemographic descriptions tend to be missing from this field’s studies (20). Our synthesis aims to incorporate this trifecta intersection of age (emerging adulthood), health status (type 1 diabetes), and SES (e.g., racial and ethnic minority group members living in poverty), if possible.
Our preliminary overview of this field found nine potentially relevant systematic reviews and/or meta-analyses published during the past 10 years (20–28). Perhaps because type 1 diabetes is typically diagnosed in children and youths (29–31), four of those reviews concerned people in those age-groups (21–24). The five others either reported on psychosocial interventions without accounting for age, or confounded outcomes among people with type 1 diabetes and those with type 2 diabetes, or both (20,25–28). In aggregate, these reviews of more than 100 randomized controlled trials (RCTs) or quasi-experiments found strongly suggestive evidence for the preventive potential of diverse psychosocial interventions. However, none has yet synthesized knowledge on the effectiveness of psychosocial interventions specifically for emergent adults with type 1 diabetes. This one will.
A final background note further underscores the need for this systematic review and meta-analysis. Our preparatory scoping review found a heuristically influential study out of the Joslin Diabetes Center in Boston, Mass. (32). A small pre-experiment of a modestly resourced, clinical psychologist–led, five-session support group preliminarily estimated huge benefits on glycemic control (d = 1.32) and psychosocial relief (d = 1.36) among emergent adults with type 1 diabetes (Table 1). Such effects will be described more completely in the methods section; however, these outcomes indicate that about 9 out of every 10 of the support group participants had improved glucose control and reduced burden at 5-month follow-up than they had before experiencing the intervention. This finding provocatively suggests the remarkable preventive potential of psychosocial interventions with such emergent adults. However, notwithstanding the well-known limitations of pre-experiments, this one studied very few mostly white and otherwise socioeconomically privileged participants, suggesting that its hopeful findings are likely best thought of as screened hypotheses. The purpose of this systematic review is to enhance knowledge about psychosocial interventions and their effectiveness to facilitate glycemic control and to alleviate distress among emergent adults with type 1 diabetes.
TABLE 1.
Characteristics and Outcomes of a Pre-Experiment
| Study, Location | Length of Follow-Up | Intervention | Sample Characteristics | Pre-/Post-Analytic Samples | Outcome Measures | d |
|---|---|---|---|---|---|---|
| Markowitz and Laffel (32), Boston, Mass. | 5 months | Support group* | Aged 18–30 years; 93% female; 92% white; 86% university education | 15 | Glycemic control† | 1.32 |
| 12 | ||||||
| 15 | Burden and self-care‡ | 1.36 | ||||
| 15 |
Five monthly sessions led by a clinical psychologist.
A1C test: average plasma glucose concentration over 8–12 weeks.
PAID scale and Self-Care Inventory.
Design and Methods
Data Sources and Searches
We systematically searched for studies of emergent adults aged 15–30 years with type 1 diabetes who received any psychosocial intervention. Psychosocial interventions are defined here as any intervention in which “interpersonal or informational activities, techniques, or strategies target biological, behavioral, cognitive, emotional, interpersonal, social or environmental factors with the aim of improving health functioning and well-being” (33). Studies included in the meta-analysis must have reported use of a nonrandomized comparison or a randomized control group with any standardized psychosocial measures for which findings were reported in such a way that a psychosocial intervention effect size was calculable. The potential moderating effects of key sociodemographic (e.g., race/ethnicity and SES) and intervention characteristics (e.g., type, intensity, and duration of interventions) were explored to the extent possible.
Research literature databases were searched for articles published from 1 January 1985 to 31 October 2018. The baseline date was selected to include this field’s watershed period, when all of the following phenomena were recognized: 1) the risk for mental health challenges among people with type 1 diabetes, 2) the need for transition services from pediatric to adult diabetes care, and 3) emergent adulthood as a developmental period. We searched for published research and grey, or unpublished/non–peer-reviewed, research to provide control for publication bias. Core health and social-behavioral sciences databases were searched. These included CINAHL Complete, ProQuest Nursing & Allied Health Database, Cochrane Register of Controlled Trials, EBM Reviews, PubMed/Medline, PsychINFO, Social Work Abstracts, and Social Services Abstracts. ProQuest Dissertations and Theses Global, Web of Science Conference Proceedings Citation Indexes–Science and Social Sciences & Humanities, and Google Scholar were also searched. The following keyword search scheme was used: (type 1 or I) and diabetes and (intervention or treatment or therapy or psychoeducation or support or social work or psychology or psychiatry or nursing) and (emerg* or young or early [15–30 years of age]) and adult (34,35). Relevant retrieved reviews and bibliographies were searched for eligible primary studies.
Study Selection
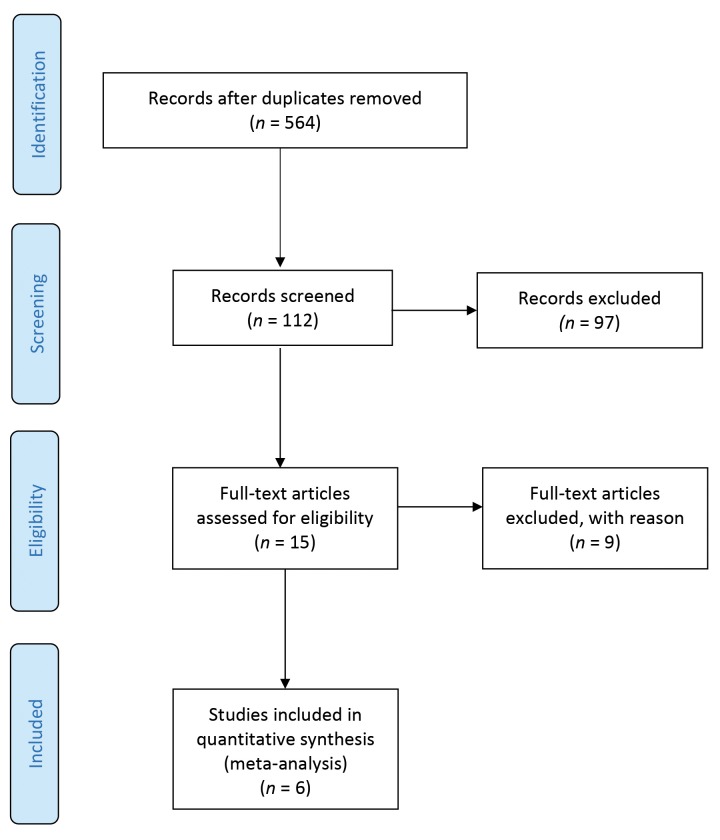
Systematic database searches and deduplication generated 564 distinct titles. Potentially eligible studies were first screened by one reviewer based on their titles; 15 full-text articles— principally their abstracts and methods sections—were reviewed, and decisions regarding whether to include were made by both reviewers. We discussed and reached agreement on the few discrepancies.
As the study selection process unfolded, it became clear that few studies would be eligible for inclusion; consequently, we would have a nearly “empty” review (36). Any otherwise eligible study in which the vast majority (≥75%) of participants were within the targeted age range of 15–30 years was also included to liberalize the age criterion somewhat. Six independent studies met all of the inclusion criteria for meta-analysis (37–42). A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram outlining the study selection process is displayed in Figure 1 (43,44).
FIGURE 1.
PRISMA flow diagram for the systematic review process.
Data Extraction and Quality Assessment
Both reviewers abstracted study characteristics independently from full primary study articles. After discussion and resolution of discrepancies, agreement was 100%. This preliminary meta-analysis explored the following descriptive or potentially moderating study characteristics: publication year and study place; participants’ age, sex, race/ethnicity, and SES/health insurance status distributions; interveners’ professional credentials and intervention intensity, duration, and program endowment/total contact hours; and study sample sizes, research design typology, type comparison/control condition, type/validity of measures, length of follow-up, and attrition rates. To gain an individualized view of each study, study quality and its potential relationship to study outcomes were assessed separately rather than computing study quality summary scores (45).
Data Synthesis and Analysis
The longest follow-up measurements for each independent study were included in the meta-analysis. Although we initially searched for studies that reported a standardized psychosocial measure, all six of the studies ultimately included also reported a standardized biological measure (A1C). Such measures of glycemic control and psychosocial status were reported separately. Each study could contribute only once to each hypothesis test. If a primary study provided three outcomes all related to the same hypothesis (e.g., three psychosocial outcomes of anxiety, depression, and self-esteem), they were pooled so that that study would contribute one data point for that meta-analytic hypothesis test.
Cohen’s d was the central effect size statistic. It allows for conversions of various measurement scales into a common metric for straightforward comparisons across or between studies. It can be calculated directly from group means (d = M1 – M2 / (SD1 + SD2) / 2) or derived from other parametric or nonparametric statistics (46). To provide practical interpretations, d values were converted to Cohen’s U3 statistics (47). U3 is intuitively appealing because it compares all participants’ scores in the intervention group to the typical participant’s score in the control/comparison group at follow-up, putting the emphasis on people rather than statistics and therefore more readily informing clinical and policy decisions. For example, a U3 of 80% resulting from the comparison of a group of emergent adults with type 1 diabetes who received a psychosocial intervention versus otherwise similar emergent adults who received usual care on a standardized measure of anxiety would be interpreted as follows: 8 of every 10 of the people in the psychosocial intervention group scored lower on anxiety at follow-up than did the typical person in the usual care group. The meta-analysis pooled fixed study effects weighted by the number of participants who did not drop out, giving greater weight to studies with more participants. Statistical significance was estimated with 95% CIs, and effect distributions were subsequently tested for homogeneity with Cochran’s Q statistic (47). A χ2 distribution was used to test whether the variability of effects was greater than what could have been expected by sampling error alone.
Results
Sample Description
Descriptive characteristics and outcomes of the six included studies (four RCTs and two quasi-experiments) are shown in Table 2. The studies had a follow-up period of 6–18 months and were carried out in Copenhagen, Denmark; Sydney, Australia; or Los Angeles, Calif. Three of the Los Angeles–based studies included the same psychosocial intervention participants, but used unique comparison groups or interventions. These were therefore treated as unique hypothesis tests, so they were treated as independent studies in the meta-analysis.
TABLE 2.
Characteristics and Outcomes of Comparative Studies Included in the Meta-Analysis
| Study, Location | Design, Length of Follow-Up | Treatment, Comparison Condition | Sample Characteristics | Analytic Samples | Outcome Measures | d |
|---|---|---|---|---|---|---|
| Steinbeck, et al. (39), Sydney, Australia | RCT, 12 months | Comprehensive transition,* standard clinical practice | Age 17–18 years, 54% female | 9 | Glycemic control† | 1.81 |
| 9 | ||||||
| 9 | Self-worth‡ | 0.07 | ||||
| 5 | ||||||
| Zoffmann et al. (40), Copenhagen, Denmark | RCT, 18 months | Guided self-determination,§ usual care waiting list | Age 18–35 years, 50% female | 134 | Glycemic control† | 0.26 |
| 66 | ||||||
| 92 | Burden, well-being, self-esteem, and six others |,¶ | 0.23 | ||||
| 59 | ||||||
| Pyatak et al. (41), Los Angeles, Calif. | RCT, 6 months | Individualized activity analysis,# attention control** | Age 18–30 years, 63% female,90% non-white | 38 | Glycemic control† | 0.50 |
| 37 | ||||||
| 34 | Well-being (three measures), depression, and seven others†† | 0.21 | ||||
| 37 | ||||||
| Weigensberg et al. (42), Los Angeles, Calif. | RCT, 12 months | Diabetes empowerment council,‡‡ non-attendees | Mean age 19.8 years (SD 1.1 years), 49% female, 62% Hispanic | 9 | Glycemic control† | 0.33 |
| 22 | ||||||
| 8 | Well-being (three measures), depression, and two others§§ | 0.38 | ||||
| 18 | ||||||
| Sequeira et al. (38), Los Angeles, Calif. | Quasi-experiment, 12 months | Structured transition,|| usual care | Age 19–25 years, 44% female, 75% non-white, 67% Medicaid | 43 | Glycemic control† | 0.18 |
| 25 | ||||||
| 37 | Well-being (two measures), depression, and four others¶¶ | 0.27 | ||||
| 26 | ||||||
| Pyatak et al. (37), Los Angeles, Calif. | Quasi-experiment, 12 months | Structured transition,|| lapsed care | Age 19–25 years, 47% female, 92% non-white, 89% Medicaid | 43 | Glycemic control† | 0.64 |
| 15 | ||||||
| 37 | Well-being (two measures), depression, and four others## | 0.37 | ||||
| 11 | ||||||
| Meta-analytic statistics | Sample-weighted d | 0.31 | ||||
| 95% CI around the weighted d | (0.17– 0.45) | |||||
Four 5- to 20-minute telephone support sessions with a transition coordinator at weeks 1 and 3 and months 6 and 12.
A1C test: average plasma glucose concentration over 8–12 weeks.
Single item from the Self-Perception Profile for Adolescents.
Seven 1-hour individual or 2.5-hour small-group sessions with two to seven participants led biweekly by diabetes nurse specialists.
PAID scale, WHO-5 Well-Being Index, Rosenberg Self-Esteem Scale, Self-Determination Scale—three subscales, relative autonomy with the Treatment Self-Regulation Questionnaire, autonomy support with the Health Care Climate Questionnaire and the Perceived Competence in Diabetes scale.
d associated with the PAID scale was 0.52.
Seven modules delivered individually to meet participant goals.
Initial home visit, followed by 11 follow-up telephone calls.
Audit of Diabetes-Dependent Quality of Life, PAID scale, Satisfaction With Life Scale (SWLS), Patient Health Questionnaire-8, Diabetes Empowerment Scale-Short Form (DES-SF), Diabetes Knowledge Questionnaire, Diabetes Problem-Solving Inventory, Self-Report Behavioral Automaticity Index, Participation Objective, Participation Subjective Summary of Diabetes Self-Care Activities—two subscales, frequency of self-monitoring of blood glucose and medication adherence.
Twelve 1.5-hour small-group sessions every 3–4 weeks.
General Well-Being Index, Arizona Integrative Outcomes Scales—24-hour and 1-month well-being measures, Patient Health Questionnaire-9 (PHQ-9), SWLS, and Perceived Stress Scale.
Let’s Empower and Prepare (LEAP) program: tailored education at quarterly visits, case management, and web-based peer support via a private social network.
Twenty-four-hour and 1-month well-being measures and perceived stress, life satisfaction, and diabetes knowledge and empowerment (standardized or unique operational measures not reported).
DES-SF, Diabetes Knowledge Test, adapted Perceived Stress Scale (for increased comprehension by Hispanic adolescents), PHQ-9, SWLS, and Arizona Integrative Outcomes Scales—24-hour and 1-month well-being measures.
One intervention was essentially a brief, telephone-based case management program with ∼1 hour of total per-patient professional contact time (Table 2, row 1) (39). Not surprisingly, that intervention had no statistical or practical effect on participants’ psychosocial status, a single-item measure of their sense of self-worth (d = 0.07). Moving down the table, the next listed intervention seemed naturalistically psychotherapeutic, if modestly endowed. It provided seven individual or small-group counseling sessions, with professional-patient contact times (with diabetes nurse specialists) of 7–18 hours (40). The next intervention provided individualized assessment and administration of manualized modules, delivering 10–16 hours of contact time with an occupational therapist (41). The final RCT used guided imagery, storytelling, discussion, and other activities to encourage learning and reflection in a small group–based intervention that provided 7–18 hours of contact time (42). This study and the two quasi-experiments were based in Los Angeles, and participants were predominantly Hispanic individuals who were largely Medicaid-insured. The remaining two interventions studied the same, seemingly much more resourceful, direct case management–based intervention augmented with Web-based social supports (37,38). It should be noted that the last study listed in Table 2 probably overestimated its intervention’s benefits, since they were based on an arguably quite liberal comparison with a group of emergent adults with type 1 diabetes whose routine care had lapsed.
As for research methods, the four RCTs and the two quasi-experiments overall were not very well controlled. They were generally small trials, potentially prone to selection bias. For example, the typical follow-up assessment of psychosocial status involved fewer than 40 intervention participants. Moreover, the typical and aggregated study dropout rates were 23% (data not shown). However, this small group of studies seemed to have one fairly consistent strength. With one above noted single-item exception, they were based on standardized and validated laboratory or psychometric measures. For example, all of the studies used the A1C test that measures the average plasma glucose concentration over the previous 8–12 weeks to assess glycemic control. Also, assessments of prevalent psychosocial statuses such as burden (and interrelated constructs such as anxiety, depression, and well-being) used well-validated measures such as the Problem Areas in Diabetes (PAID) scale.
Meta-Analytic Findings
There seemed to be some variability in the 12 reported outcomes, as d values ranged from 0.07 to 1.81. However, the nonsignificant heterogeneity analysis indicated that the total variation among the 12 effects could be explained by sampling error (χ2[11] = 11.15, P = 0.43). Such demonstrated homogeneity made separate biological-psychosocial analyses, as well as exploratory moderator analyses, moot. Consequently, one pooled meta-analysis was performed. The sample-weighted, pooled d value was 0.31 (95% CI 0.17–0.45). Because the 95% CI did not include the null value of 0.00, the aggregated difference between the pooled psychosocial intervention and control/comparison groups was statistically significant (P <0.05). It would seem clinically significant, as well; nearly two-thirds of the treated participants (U3 = 62.2%) were doing better at follow-up than the typical control group participant on both measures of glycemic control and psychosocial status.
Discussion
This near-empty systematic review and exploratory meta-analysis preliminarily suggests that psychosocial interventions providing emotional and skill-building support may significantly reduce psychological distress (e.g., burden, depression, and anxiety) and enhance glycemic control among at-risk emerging adults with type 1 diabetes as they transition from pediatric to adult diabetes care. This synthesis allowed for a very tentative estimate that perhaps as many as two-thirds of so-treated young adults with type 1 diabetes do better than the typical, otherwise similar, untreated person on both glycemic control and psychosocial status. Uncovering such potential ways to manage stressors and reduce the negative effects of living with a serious chronic illness may help to realize opportunities to reduce incidences of anxiety disorders, major depression, and suicidality and ultimately to prevent suicides among emergent adults while maintaining excellent glycemic control.
The limited number of methodologically restricted studies that met our humble inclusion criteria, however, indicates that there is much work to be done in this field. In fact, we found only six relevant study reports, including four RCTs and two quasi-experiments. All were quite constrained. They assessed telephone-based case management, individualized treatment modules, and small-group counseling interventions that were modestly endowed, ranging from only 1 to 18 professional contact hours. In fact, we found no adequately powered, well-controlled randomized trials of arguably well-resourced psychosocial interventions. Using these more conservative hypothetical inclusion criteria would have produced an empty review. Consequently, our meta-analysis was exploratory, and its suggested benefits on glycemic control and mental health are preliminary. Given the noted limits of the six included primary studies, as well as the near-empty status of this systematic review, such are best considered developed hypotheses awaiting more rigorous future research testing.
Our practice experience suggested that this field’s seemingly atheoretical, psychosocial interventions are probably grossly under-resourced as well. We began to affirm this hunch with a scoping review of related fields of practice (i.e., psychosocial interventions with adolescents through emerging adults with other chronic health conditions such as asthma, cancer, cystic fibrosis, sickle cell disease, end-stage renal disease, and others). We wondered what specific psychosocial methods have been used in those fields to the best effects.
Our initial informal scope quickly found >20 relevant systematic reviews of hundreds of studies. Two exemplary reviews, along with two relevant case studies are cited here (48–51). Our initial map of this very broad field exposed four trends: 1) prevention or alleviation of anxiety and depression seem to be unifying constructions at its center; 2) professional and peer contact, emotional support, skill development, and empowerment are prevalently suggested; 3) the most prevalent traditional (cognitive behavioral) and contemporary (mindfulness) interventions seem the most promising; and 4) these interventions probably ought to be provided to at-risk emergent adults with chronic diseases at much larger doses than has been commonly practiced.
The most prevalent and seemingly effective cognitive behavioral and mindfulness interventions in these and allied fields, for example, provide 20–30 contact hours or more in therapeutic professional-patient/client milieus (52,53). A full such scoping review, perhaps culminating with an overview of systematic reviews, may help to plan the next generation of more theoretically coherent and methodologically comprehensive psychosocial interventions for work with emergent adults with type 1 diabetes.
Limitations and Future Research Needs
One, and perhaps all, of the trials included in this meta-analysis might be better characterized as randomized pilot trials than true RCTs. Because they typically had <40 participants in their psychosocial intervention study group at follow-up, such small samples probably could not have ensured the kind of confident control for even unanticipated confounding that one hopes to achieve through randomization. What is called for is a more confident knowledge base that could be produced by large, perhaps multisite, RCTs. These should be statistically powered by ample samples of emergent adults with type 1 diabetes that are sufficient to allow the detection of modest but clinically significant between-group differences with confidence. For example, using fairly standard statistical criteria (two-tailed α = 0.05; power1-β = 0.80) samples of 150–300 participants in each study group, intervention and control, would be required to detect between-group differences characterized by d values in the neighborhood of 0.30 (54). Another seemingly prevalent limitation of most of the included studies was their apparent lack of blinding. Clearly the interveners, often the investigators and authors, cannot be blind to participants’ group status, but research assistant assessors certainly can be. Future studies should be amply funded, allowing for the staffing, training, and follow-up procedural supports needed to ensure unbiased participant assessments and high study completion rates.
As social work practitioners, we were initially very interested in the potential moderating influences of participants’ racial and ethnic group and SES. However, only one research group (three studies) described the ethnic distribution of their samples. Based in southern California, the vast majority of that group’s participants were Hispanic people, seemingly of relatively low SES (most were Medicaid-covered). Future quasi-experiments or cohorts—preferably prospective cohorts—that transcend mere laboratory and psychometric outcomes should test the moderating influence of race/ethnicity and SES and report their intervention effects separately. Large cohorts of >1,000 participants per study group will be needed (54) to be able to powerfully detect the most meaningful morbid and mortal outcomes (e.g., clinical depression diagnoses and suicides) while adjusting for myriad potential confounders.
Conclusion
This meta-analysis provides some suggestion that psychosocial interventions, including telephone-based case management, individualized treatment modules, and small-group counseling interventions, may diminish burden, depression, and anxiety and enhance glycemic control among emerging adults with type 1 diabetes as they transition from adolescence to adulthood. However, on the synthesis of confident, clinically significant knowledge, this was fundamentally an empty review. It tentatively proposed benefits across diverse, but typically poorly endowed, psychosocial interventions on glycemic control and mental health. It then identified a path to more confident knowledge, calling for adequately powered RCTs of longer-term effects of well-endowed psychosocial interventions on practically significant morbid and mortal outcomes.
Acknowledgments
The authors thank Sharon Munro of Leddy Library, University of Windsor, for library science support.
Funding
This study was supported in part by an Ontario Graduate Scholarship.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
R.R.J. conceptualized the study and led the analysis and writing. K.M.G. supervised the analysis and writing. Both authors designed the study, interpreted its findings, and approved the final manuscript. R.R.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for its integrity and the accuracy of the data analysis.
References
- 1.Arnett JJ. Emerging Adulthood: The Winding Road from the Late Teens Through the Twenties. 2nd ed. New York, Oxford University Press, 2014 [Google Scholar]
- 2.Findlay L. Depression and suicidal ideation among Canadians aged 15–24. Health Rep 2017;28:3–11 [PubMed] [Google Scholar]
- 3.Roh BR, Jung EH, Hong HJ. A comparative study of suicide rates among 10–19-year-olds in 29 OECD countries. Psychiatry Investig 2018;15:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weissberg-Benchell J, Wolpert H, Anderson BJ. Transitioning from pediatric to adult care: a new approach to the post-adolescent young person with type 1 diabetes. Diabetes Care 2007;30:2441–2446 [DOI] [PubMed] [Google Scholar]
- 5.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016;16:9. [DOI] [PubMed] [Google Scholar]
- 6.Rassart J, Luyckz K, Bijttebier P, Berg CA, Moons P, Weets I. Psychosocial functioning and glycemic control in emerging adults with type 1 diabetes: a 5-year follow-up study. Health Psychol 2015;34:1058–1065 [DOI] [PubMed] [Google Scholar]
- 7.Palladino DK, Helgeson VS, Reynolds KA, Becker DJ, Siminerio LM, Escobar O. Emerging adults with type 1 diabetes: a comparison to peers without diabetes. J Pediatr Psychol 2013;38:506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maia AC, Braga AA, Brouwers A, Nardi AE, Oliveira e Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry 2012;53:1169–1173 [DOI] [PubMed] [Google Scholar]
- 9.Johnson B, Eiser C, Young V, Brierley S, Heller S. Prevalence of depression among young people with type 1 diabetes: a systematic review. Diabet Med 2012;30:199–208 [DOI] [PubMed] [Google Scholar]
- 10.Northam EA, Lin A, Finch S, Werther GA, Cameron FJ. Psychosocial well-being and functional outcomes in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2010;33:1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrak F, Hardt J, Wittchen HU, et al. . Prevalence of psychiatric disorders in an onset cohort of adults with type 1 diabetes. Diabetes Metab Res Rev 2003;19:216–222 [DOI] [PubMed] [Google Scholar]
- 12.Anderson RJ, Clouse RE, Freedland KE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 13.Matlock KA, Yayah Jones NH, Corathers SD, Kichler JC. Clinical and psychosocial factors associated with suicidal ideation in adolescents with type 1 diabetes. J Adolesc Health 2017;61:461–477 [DOI] [PubMed] [Google Scholar]
- 14.Pompili M, Forte A, Lester D, et al. . Suicide risk in type 1 diabetes mellitus: a systematic review. J Psychosom Res 2014;76:352–360 [DOI] [PubMed] [Google Scholar]
- 15.McBrien KA, Manns BJ, Hemmelgarn BR, et al. . The association between sociodemographics and clinical characteristics and poor glycemic control: a longitudinal cohort study. Diabet Med 2016;33:1499–1507 [DOI] [PubMed] [Google Scholar]
- 16.Rechenberg K, Whittemore R, Grey M, et al. . Contribution of income to self-management and health outcomes in pediatric type 1 diabetes. Pediatr Diabetes 2016;17:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caccavale LJ, Weaver P, Chen R, Streisand R, Holmes CS. Family density and SES related to diabetes management and glycemic control in adolescents with type 1 diabetes. J Pediatr Psychol 2015;40:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randall L, Peng L, Begovic J, et al. . Recurrent diabetic ketoacidosis in inner-city minority patients. Diabetes Care 2011;34:1891–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A, Roy M, Janal M. Suicide attempts and ideation in African-American type 1 diabetic patients. Psychiatry Res 2010;179:53–56 [DOI] [PubMed] [Google Scholar]
- 20.Findley MK, Cha E, Wong E, Faulkner MS. A systematic review of transitional care for emerging adults with diabetes. J Pediatr Nurs 2015;30:e47–e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charalampopoulos D, Hesketh KR, Amin R, Paes VM, Viner RM, Stephenson T. Psycho-educational interventions for children and young people with type 1 diabetes in the UK: how effective are they? A systematic review and meta-analysis. PLoS One 2017;12:e0179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abualula NA, Jacobsen KH, Milligan RA, Rodan MF, Conn VS. Evaluating diabetes educational interventions with a skill development component in adolescents with type 1 diabetes: a systematic review focusing on quality of life. Diabetes Educ 2016;42:515–528 [DOI] [PubMed] [Google Scholar]
- 23.Ayling K, Brierley S, Johnson B, Heller S, Eiser C. Efficacy of theory-based interventions for young people with type 1 diabetes: a systematic review and meta-analysis. Br J Health Psychol 2015;20:428–446 [DOI] [PubMed] [Google Scholar]
- 24.Dudzik J, Golobich J. Effective Psychoeducational Interventions for Young Individuals with Diabetes: A Systematic Literature Review. Duluth, Minn., The College of St. Scholastica, 2011 [Google Scholar]
- 25.Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based interventions on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol 2017:22:965–983 [DOI] [PubMed] [Google Scholar]
- 26.O’Hara MC, Hynes L, O’Donnell M, et al. . A systematic review of interventions to improve outcomes for young adults with type 1 diabetes. Diabet Med 2017;34:753–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott S. Cognitive behavioural therapy and glycaemic control in diabetes mellitus. Practical Diabetes 2012;29:67–71 [Google Scholar]
- 28.Harkness E, MacDonald W, Valderas J, Coventry P, Gask L, Bower P. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: a systematic review and meta-analysis. Diabetes Care 2010;33:926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harjutsalo V, Lammi N, Karvonen M, Groop PH. Age at onset of type 1 diabetes in parents and recurrence risk in offspring. Diabetes 2010;59:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Chapter 1: Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soltesz G, Patterson CC, Dahlquist G, et al. . Worldwide childhood type 1 diabetes incidence: what can we learn from epidemiology? Pediatr Diabetes 2007;8:6–14 [DOI] [PubMed] [Google Scholar]
- 32.Markowitz JT, Laffel LMB. Transitions in care: support group for young adults with type 1 diabetes. Diabet Med 2012;29:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine, Board of Health Sciences Policy , Committee on Developing Evidence-Based Standards for Psychosocial Interventions for Mental Disorders. Introduction. In Psychosocial Interventions for Mental and Substance Use Disorders: A Framework for Establishing Evidence-Based Standards. England MJ, Butler AS, Gonzalez ML, Eds. Washington, D.C., National Academies Press, 2015, p. 21–46 [PubMed] [Google Scholar]
- 34.Schilling LS, Knafl KA, Grey M. Changing patterns of self-management in youth with type I diabetes. J Pediatr Nurs 2006;21:412–424 [DOI] [PubMed] [Google Scholar]
- 35.Hynes L, Byrne M, Dinneen SF, McGuire BE, O’Donnell M, McSharry J. Barriers and facilitators associated with attendance at hospital diabetes clinics among young adults (15–30 years) with type 1 diabetes mellitus: a systematic review. Pediatr Diabetes 2016;17:509–518 [DOI] [PubMed] [Google Scholar]
- 36.Yaffe J, Montgomery P, Hopewell S, Shepard LD. Empty reviews: a description and consideration of Cochrane systematic reviews with no included studies. PLoS One 2012;7:e36626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyatak EA, Sequeira PA, Vigen CP, et al. . Clinical and psychosocial outcomes of a structured transition program among young adults with type 1 diabetes. J Adolesc Health 2017;60:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sequeira PA, Pyatak EA, Weigensberg MJ, et al. . Let’s Empower and Prepare (LEAP): evaluation of a structured transition program for young adults with type 1 diabetes. Diabetes Care 2015;38:1412–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbeck KS, Shrewsbury VA, Harvey V, et al. . A pilot randomized controlled trial of a post-discharge program to support emerging adults with type 1 diabetes mellitus transition from pediatric to adult care. Pediatr Diabetes 2015;16:634–639 [DOI] [PubMed] [Google Scholar]
- 40.Zoffmann V, Vistisen D, Due-Christensen M. Flexible guided self‐determination intervention for younger adults with poorly controlled type 1 diabetes, decreased HbA1c and psychosocial distress in women but not in men: a real‐life RCT. Diabet Med 2015;32:1239–1246 [DOI] [PubMed] [Google Scholar]
- 41.Pyatak E, Caradang K, Vigen C, et al. . Occupational therapy intervention improves glycaemic control and quality of life among young adults with diabetes: the Resilient, Empowered, Active Living with Diabetes (REAL Diabetes) randomized controlled trial. Diabetes Care 2018:41:696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigensberg M, Vigen C, Sequeira P, et al. . Diabetes empowerment council: integrative pilot intervention for transitioning young adults with type 1 diabetes. Glob Adv Health Med 2018:7: 2164956118761808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev 2016;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463–471 [DOI] [PubMed] [Google Scholar]
- 46.Cooper H. Research Synthesis and Meta-Analysis: A Step-by-Step Approach. 5th ed Thousand Oaks, Calif, Sage, 2017 [Google Scholar]
- 47.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, N.J., Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 48.Bennett S, Shafran R, Coughtrey A, Walker S, Heyman I. Psychological interventions for mental health disorders in children with chronic physical illness: a systematic review. Arch Dis Child 2015;100:308–316 [DOI] [PubMed] [Google Scholar]
- 49.Sansom-Daly UM, Peate M, Wakefield CE, Bryant RA, Cohn RJ. A systematic review of psychological interventions for adolescents and young adults living with chronic illness. Health Psychol 2012;31:380–393 [DOI] [PubMed] [Google Scholar]
- 50.Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol 2012;30:1221–1226 [DOI] [PubMed] [Google Scholar]
- 51.Cabness J, Miller C, Flowers K. Promoting resilience in ESRD: evaluation of a group cognitive-behavioral intervention for patients on hemodialysis. J Nephrol Soc Work 2006;25:30–36 [Google Scholar]
- 52.Escobar KM, Gorey KM. Cognitive behavioral interventions for depression among Hispanic people: promising meta-analytic evidence for deep cultural adaptations. Soc Work Ment Health 2018;16:746–758 [Google Scholar]
- 53.Singh SK, Gorey KM. Relative effectiveness of mindfulness and cognitive behavioral interventions for anxiety disorders: meta-analytic review. Soc Work Ment Health 2018;16:238–251 [Google Scholar]
- 54.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd ed. Hoboken, N.J., Wiley, 2003 [Google Scholar]