Abstract
IN BRIEF The traditional approach to integrating new therapies involves long, expensive roadmaps with evidence generation required for multiple stakeholders, most notably regulators and clinicians. More recently, new technologies such as insulin delivery systems and continuous glucose monitoring devices have become mainstream without complete clinical evidence being available when they were first introduced. There is tremendous enthusiasm from investors, industry, and people with diabetes regarding the potential of digital health to add value to diabetes care, and this enthusiasm exists despite a paucity of high-quality clinical evidence from traditional randomized clinical trials. Moreover, the potential of diabetes digital health technologies has been recognized by the U.S. Food and Drug Administration and other regulators, who are changing their approaches to allow easier, earlier access to diabetes software and devices. This wager that digital health will add value makes sense.
Pascal’s Wager was a suggestion posed by the French philosopher Blaise Pascal that even though the existence of God cannot be determined through reason, a person should wager that God exists because there is everything to gain and nothing to lose. Almost a decade ago, we suggested (wagered) that you could apply similar reasoning to technologies such as continuous glucose monitoring (CGM) systems being created for people with diabetes: the introduction of these technologies into clinical practice would be beneficial beyond the available evidence at that time (i.e., reason) (1). Going forward, we believe that a similar type of reasoning is appropriate for other forms of “digital health”; these new devices, applications (apps), and software packages will provide benefits for people with diabetes in the absence of compelling evidence because there is everything to gain and nothing to lose, albeit with caveats of cost, convenience, and time burden. Our reasoning (wager) is based on considerations of 1) what is digital health, 2) what are the metrics of success for digital health, and 3) what is the value of digital health for diabetes?
What Is Digital Health?
New digital technologies, including wearable and implanted sensors and effectors, comprise part of a digital ecosystem of data-driven tools that can link people with diabetes and their care teams, enabling precision management of diabetes (2). In practical terms, a digital health product consists of a wearable (or implanted) sensor with wireless communication, a smartphone to receive the information, and software (referred to a “mobile app”) to process the information. At present for diabetes care, a usual consequence of using a digital health product is representation or interpretation of the data for the user. In some circumstances, there may also be decision support or even an automatic effector action (i.e., an automatic response to a trigger created by the software).
The aim of creating and using digital health technologies is to facilitate the so-called “four purposes” of health care, namely diagnosis, monitoring, treatment, and prevention, leading to four types of outcomes. For diabetes care, the first outcome is increased knowledge of factors affecting diabetes control. Second is support for an individual to move toward a healthier lifestyle that is likely to lead to improved outcomes. Third is support for healthy engagement with a mutually agreed upon treatment regimen so that a person with diabetes will accrue the maximum benefit from this regimen. Fourth is reduction in the personal time burden for self-management. For example, when a smart sensor (defined as a device taking input from the environment and using computer resources to trigger predefined functions upon detection of specific input) is connected directly to an actuator (defined as a device with integrated software that acts in response to electronic control from a sensor), then the sensor is said to control the actuator and therefore can be automated.
Digital health data are crucially important to support the modern precision medicine paradigm for directing medical treatment to the individual anatomy, biochemistry, and physiology of each patient (3). In simple terms, precision medicine involves ascertaining, combining, and applying four types of information into a specific picture of each individual: 1) a phenotypic assessment, 2) a sensor-based behavioral assessment, 3) genetic information, and 4) “-omics” (e.g., genomics, proteomics, or metabolomics) expressions of genes. Connected wearable sensors and mobile apps, which are basic components of digital health systems, inform the first and second items. In diseases such as diabetes, for which individualized genetic information and genomic-based pharmacotherapy are not readily accessible and behavior and physiological status are changing throughout the day, these digital health tools are particularly valuable for describing the features of a patient’s health. Furthermore, constantly updating smart sensor information can provide an evolving real-time phenotypic picture of any patient.
The digital health paradigm of continuous observation provides a new dimension for understanding an individual’s medical status based on multiple sensor-enabled physiological measurements that are analyzed and stored in the Cloud (Internet). Digital health tools with real-time monitoring capabilities can greatly supplement single data points that represent aggregated data, such as A1C, mean achieved glucose, time in range, typical daily insulin dose, and insulin sensitivity (4). The use of real-time knowledge can then empower better real-time decisions.
Ultimately, judgment of digital health tools will be based on their ability to deliver improved outcomes that matter to people with diabetes, clinicians, and other stakeholders. Ideally, these technologies should also reduce the time burden for each of these groups. In the assessment of digital health products, it is notable that the metrics of success may be different for different audiences involved in diabetes care (Table 1). Overall, digital health tools need to demonstrate usability, clinical benefit, economic benefit, adequate security, and safety to satisfy various stakeholders (5). For digital tools that can demonstrate these attributes, health care systems will have to be reconfigured to accommodate the use of digital health data. Importantly, this means that electronic health records will need to integrate digital data, clinical workflows will have to accommodate digital data, and manufacturers of digital systems will be required to follow standards to make their hardware interoperable and their data capable of integration (6).
TABLE 1.
Examples of Three Potential Metrics of Success for Digital Health Technologies Applied to Diabetes, According to Stakeholder*
| Stakeholder | Metric | Metric | Metric |
|---|---|---|---|
| People with diabetes | Less hypoglycemia | Less glucose variability | Less excess weight gain |
| Caregivers or family members of people with diabetes | Less time spent on diabetes management | Less financial burden | Fewer episodes of uncontrolled diabetes, especially severe hypoglycemia |
| Clinicians | Less time spent dealing with unscheduled visits | Less time spent on nonclinical aspects of care | More appropriate reimbursement models |
| Payers | Improved value and less cost | Reduced variation in care and less waste | Better access to and effectiveness of preventive measures |
| Industry | Wider adoption of value-based health care | Lower rates of adverse clinical outcomes | Lower research and development costs |
This is not an exhaustive list.
What Are the Metrics of Success for Digital Health and Diabetes?
A 2018 review of the efficacy, usability, and features of commercially available smartphone apps for diabetes self-management published by the Agency for Healthcare Research and Quality (AHRQ) of the U.S. Department of Health and Human Services reached four conclusions (Table 2) (7). Overall, studies of only five apps showed a clinically meaningful reduction in A1C of at least 0.5 percentage points when compared to usual care. Of those five, two were for type 1 diabetes and three were for type 2 diabetes. Furthermore, only a few commercially available apps had clinical evidence supporting improved glycemic control, and the impact of the apps could not be distinguished from the concomitant effect of additional support from a health care provider. These A1C findings were similar to average A1C decreases (compared to controls) in randomized controlled trials of diabetes mobile apps noted elsewhere (8,9). An additional limitation of the evidence is that no trials have yet reported outcomes beyond 1 year. It is noteworthy that, currently, 21% of users abandon an app after one use (10). For downloaded health-related apps, about half of users give up because of the amount of time taken to enter data, loss of interest, hidden costs, challenges with understanding how to use them, and concerns about data-sharing (11).
TABLE 2.
Four Key Messages From the AHRQ Review of Mobile Apps for Diabetes Self-Management
| 1. Although hundreds of apps for diabetes self-management are commercially available, we only identified health outcomes studies on 11 apps. |
| 2. Of the 11 apps, studies showed that only 5 were associated with clinically significant improvements in A1C, an important clinical test for monitoring diabetes (for type 1 diabetes, Glucose Buddy and Diabeo Telesage; for type 2 diabetes, Blue Star, WellTang, and Gather Health) |
| 3. None of the studies showed patient improvements in quality of life, blood pressure, weight, or BMI. More rigorous and longer-term research studies could determine whether apps help people manage their diabetes and reduce complications. |
| 4. The studies had methodological issues. They were short (2–12 months); inconsistent in reporting of randomization, allocation, masking, and dropout analysis; and often used co-interventions that hindered interpretation of results. None of the included studies was considered to be of high quality. |
What Is the Value of Digital Health?
The digital health revolution began with the creation of technologies that focused on helping individuals adopt more healthy lifestyles, usually based on nutrition and physical activity self-monitoring. Over time, there has been a fundamental shift to expectations that the use of digital health technologies will provide meaningful and measurable personal benefits. This shift has occurred mainly due to advances in sensor technology (especially miniaturization, increased power, and improvements in aesthetics), smartphone computing capability, and most notably, the promise of artificial intelligence (AI). What has not changed is the challenge for digital health innovators to prove that their products have value.
Equation 1 can help define what value means (12). The value of a health care intervention depends on the perspective of the observer. The questions of who pays for the intervention and who receives its benefits greatly affect stakeholder perceptions of value. A calculation of the value of digital health would depend on many factors such as the current health condition of the user, the pattern of disease progression, the effectiveness of the intervention, the frequency of measurement and intervention, and the cost of measuring.
From the perspective of payers, there are few data in the literature to support the economic value of digital health for diabetes (13). From the perspective of users as consumers, the outcomes from using such technologies should have meaningful value beyond surrogate measures such as average achieved glucose and A1C. Although this type of metric is clinically important in terms of defining the risk of long-term complications, from a digital perspective, as a measure of value, it is likely to be too distant to have much relevance to users. Glucose-based outcomes such as ambulatory glucose profiles (which show a patient’s daily glucose and insulin patterns, including time in the target range and a graphic representation of excursions out of that range) and glucose management indicators using CGM data are likely to have more impact for clinicians and people with diabetes (14). Another approach could include the use of patient-reported outcome measures; these involve patients responding to questions on themes such as physical or social functioning and mental well-being, including disease-specific questions (15).
Experience of care is likely to be based on users’ experiences with the app, its financial cost (i.e., free versus subscription), and the personal time burden of incorporating the technology into daily life (i.e., adding more time to use a technology will be a negative experience). A 2018 survey by Black Book Market Research of nearly 650 health care consumers found that the digital consumer experience is of high priority, and specifically, 92% of respondents stated that improving the customer experience should be a top strategic priority for medical providers. Respondents stated that they expected to use digital tools for improved patient-provider interactions (93%) as well as improved processes for care delivery, such as virtual care access (85%), online scheduling (97%), online payment tools (92%), and online price transparency tools (94%) (16). Finally, and importantly, digital health technologies will be expected to reduce costs for a health care system and not to reduce reimbursement for clinicians.
 |
EQ. 1. Defining value in digital health. |
The U.S. Centers for Medical & Medicaid Services final 2019 physician fee schedule and quality payment program provide reimbursement for three types of connected-care services that enable providers to manage and coordinate care at home. The changes pertain to three new CPT (Current Procedural Terminology) codes that separate remote physiologic monitoring services from telehealth (17). Interpretation of CGM data was not specifically presented as an example procedure in these three new billing codes, and it is currently unclear whether this type of monitoring will be covered by these codes.
Achievement of the promise of the digital health revolution will require new approaches to clinical trial design, including the use of intention control comparators (e.g., testing a smartphone app may not need to be as a stand-alone technology but as a tool to integrate with existing care). It will also be necessary to reach a consensus on how to measure adherence to a “digital solution” (i.e., by asking what keeps people using these products and what are the relevant metrics to measure their use) (18).
The true goal for digital diabetes health is to integrate technology with, not substitute for, the health care team. Increasingly, combining new digital health tools such as wearable/portable/implantable sensors with companion software for using these technologies for monitoring, treatment, and, to a lesser extent, prevention or diagnosis of diabetes and its complications is becoming more commonplace. An extension of this trend is the use of digital technology to support people with diabetes starting and staying with prescribed therapies, but with a markedly reduced time burden.
For other stakeholders in diabetes care who represent the investment, academic, and regulatory communities, there are indications of a recent surge of interest in diabetes digital health as a model for other chronic medical conditions. Four key trends from these stakeholders are currently driving advances in digital health delivery: 1) increasing financial investment in digital health technology, 2) accelerating development of new ideas and technologies for digital health from academia and industry, 3) development of new ideas for streamlined regulation of the digital health industry by regulators such as the FDA, and 4) increasing use of real-world data collection by mobile apps for clinical trials (19).
Digital Diabetes Ecosystem
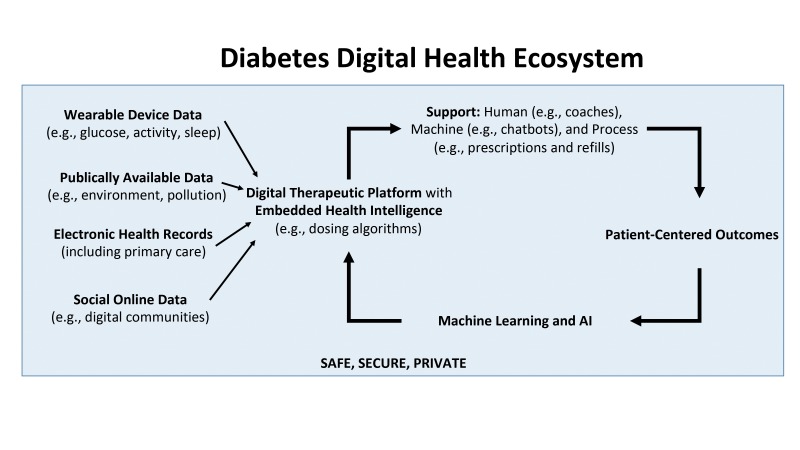
In the future, a digital diabetes ecosystem will combine the Internet of Health Things, personal health sensor data, and the use of AI and machine learning with smartphones providing continuous access to the Internet. The system will take in data from multiple sources ranging from big data sets to wearable sensing devices and also information from electronic health records and online digital communities. The digital therapeutic platform will have embedded algorithms to support diabetes care and also reduce the friction associated with self-management tasks (Figure 1).
FIGURE 1.
Structure of a digital diabetes ecosystem of the future.
Conclusion
By considering what digital health is, what the metrics of success for digital health in diabetes are, and what the value of digital health for diabetes is, one can appreciate the clinical and economic benefits of this paradigm. Digital health for diabetes consists of new smart sensors and mobile apps for measuring body processes such as continuous glucose levels. This approach to health care for diabetes has been shown to improve A1C through effects on knowledge, behavior, and, in the case of closed-loop systems, control of an insulin pump. New approaches are also being developed for sensors and for software to process and act on sensor information. A limited number of studies of the effectiveness of digital health tools for diabetes look favorable, although more extensive research will be needed to determine where these tools fit best into the health care system. We believe that digital health tools will soon also demonstrate economic value for both patients and payers. Therefore, our belief in the potential benefits of digital health can be summarized by stating that, by adopting this emerging technology, we have everything to gain and nothing to lose.
Acknowledgment
The authors acknowledge Annamarie Sucher for her expert editorial assistance.
Duality of Interest
D.K. is a medical advisor for Glooko and Vicentra and a consultant to Novo Nordisk and Sanofi. D.C.K. is a consultant for Ascensia, AstraZeneca, EOFlow, Lifecare, Merck, Novo Nordisk, Roche Diagnostics, and Voluntis. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
D.K. and D.C.K. wrote the manuscript, researched data, and reviewed/edited the manuscript. F.K. researched data and contributed to the discussion. D.C.K. is the guarantor of this work and, as such, had full access to all the data reported and takes responsibility for the integrity of the data and the accuracy of the content.
References
- 1.Kerr D, Olateju T. Pascal’s wager: combining continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Technol Ther 2010;12(Suppl. 1):S43–S50 [DOI] [PubMed] [Google Scholar]
- 2.Kerr D, Axelrod C, Hoppe C, Klonoff DC. Diabetes and technology in 2030: a utopian or dystopian future? Diabet Med 2018;35:498–503 [DOI] [PubMed] [Google Scholar]
- 3.Klonoff DC. Precision medicine for managing diabetes. J Diabetes Sci Technol 2015;9:3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagherazzi G, Ravaud P. Digital diabetes: perspectives for diabetes prevention, management and research. Diabetes Metab. Epub ahead of print on 19 September 2018 (doi: 10.1016/j.diabet.2018.08.012) [DOI] [PubMed]
- 5.Klonoff DC, Kerr D. Overcoming barriers to adoption of digital health tools for diabetes. J Diabetes Sci Technol 2018;12:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhavnani SP, Harzand A. From false-positives to technological Darwinism: controversies in digital health. Per Med 2018;15:247–250 [DOI] [PubMed] [Google Scholar]
- 7.Veazie S, Winchell K, Gilbert J, et al. . AHRQ Comparative Effectiveness Technical Briefs: Mobile Applications for Self-Management of Diabetes. Rockville, Md, Agency for Healthcare Research and Quality, 2018 [PubMed] [Google Scholar]
- 8.Bonoto BC, de Araujo VE, Godoi IP, et al. . Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth 2017;5:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016;39:2089–2095 [DOI] [PubMed] [Google Scholar]
- 10.Rodde T. 21% of users abandon an app after one use. 16 April 2018. Available from info.localytics.com/blog/21-percent-of-users-abandon-apps-after-one-use. Accessed 27 November 2018
- 11.Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR MHealth UHealth 2015;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev 2011;89:46–52, 54, 56–61 passim [PubMed] [Google Scholar]
- 13.Agnihothri S, Cui L, Delasay M, Rajan B. The value of mHealth for managing chronic conditions. Health Care Manag Sci. Epub ahead of print on 31 October 2018 (doi: 10.1007/s10729-018-9458-2) [DOI] [PubMed]
- 14.Bergenstal RM, Beck RW, Close KL, et al. . Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg FR, Peach G, Price P, Thompson MM, Hinchliffe RJ. Measures of health-related quality of life in diabetes-related foot disease: a systematic review. Diabetologia 2012;55:552–565 [DOI] [PubMed] [Google Scholar]
- 16.Black Book Market Research 19 recent healthcare tech start-ups attract instant consumer appeal: Black Book survey. 9 July 2018. Available from blackbookmarketresearch.newswire.com/news/19-recent-healthcare-tech-start-ups-attract-instant-consumer-appeal-20556737. Accessed 27 November 2018
- 17.Wicklund E. CMS to reimburse providers for remote patient monitoring services. 2 November 2018. Available from mhealthintelligence.com/news/cms-to-reimburse-providers-for-remote-patient-monitoring-services. Accessed 28 November 2018
- 18.Kerr D, Gabbay RA, Klonoff DC. Finding real value from digital diabetes health: is digital health dead or in need of resuscitation? J Diabetes Sci Technol 2018;12:911–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klonoff DC, King F, Kerr D. New opportunities for digital health to thrive. J Diabetes Sci Technol 2019;13:159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]