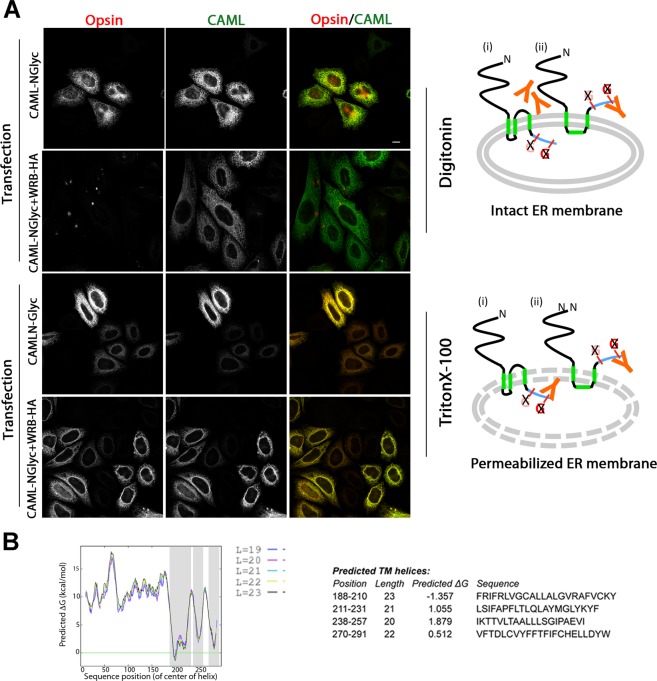
Figure 4.
In the absence of WRB-HA, the C-terminus of a portion of CAML-NGlyc molecules is exposed to the cytosol. (A) Cells, transfected with CAML-NGlyc either in the presence or absence of WRB-HA, were semi-permeabilized with digitonin before fixation. One set of samples was then processed for immunofluorescence without the addition of other detergents (upper two rows), while the second set of was processed in the presence of TX-100 (lower two rows). Samples were stained with anti-opsin and anti-CAML to visualize the C-terminal opsin epitope and the N-terminal domain of CAML-NGlyc, respectively. Scale bars 10μm. The models on the right illustrate two possible topologies of the 0-G form, distinguishable by the accessibility of the opsin epitope to antibodies: in structure (i), the 0-G form is correctly inserted with the 3-TM topology, but fails to access the OST complex; in structure (ii), TM3 fails translocation, and the opsin epitope is therefore accessible to antibodies (orange) restricted to the cytosol in SP cells. If structure (i) were correct, the opsin epitope would become accessible only in the presence of TX-100 (lower cartoon), as is indeed the case when CAML-NGlyc and WRB-HA are co-expressed. (B) Plot of ΔG for membrane integration of CAML sequence. The different colour-coded lines were obtained with different lengths (L) of the sliding window used by the algorithm, as indicated (http://dgpred.cbr.su.se/)15. Positive ΔG values predict an unfavourable free energy of membrane integration. Note the unfavourable ΔG of the second predicted TM (either res 211–231 or res 238–257, see text for further details).