Figure 3.
RAP80 Controls BRE Conformation and Prevents Dimerization
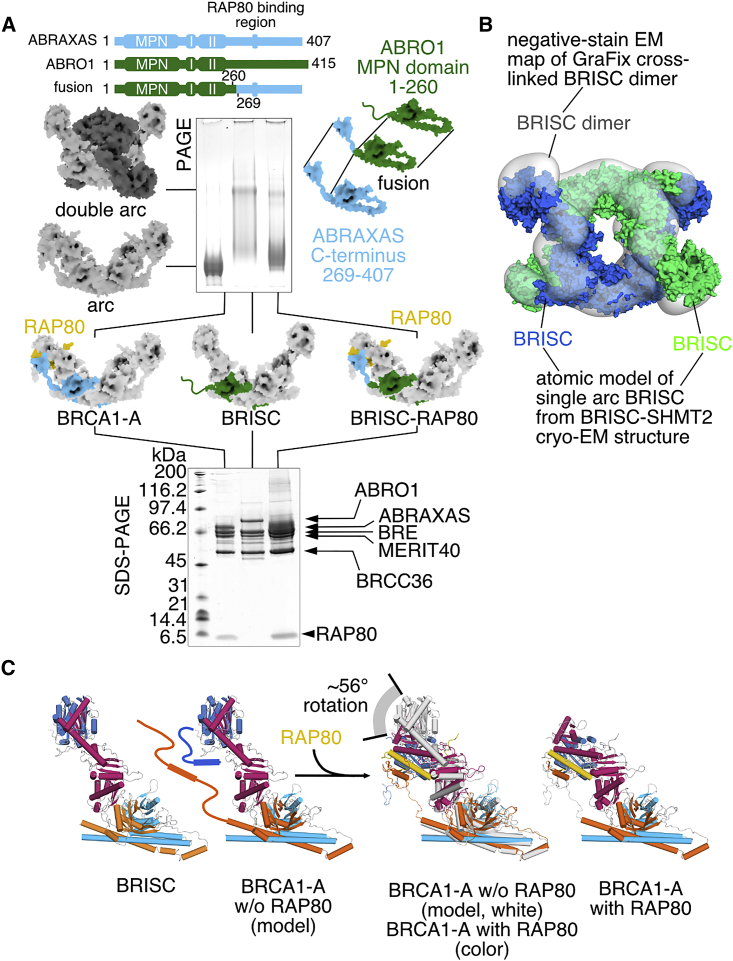
(A) A fusion scaffold protein containing residues 1–260 of mouse ABRO1 fused to residues 269–407 of mouse ABRAXAS disrupts dimerization (native PAGE) and integrates RAP80 stoichiometrically into the complex (SDS-PAGE).
(B) The structure of BRISC dimer. A pseudoatomic model of BRISC was generated by rigid-body fitting of the atomic BRISC model derived from the cryo-EM structure determination of BRISC-SHMT2α complex into the density map of BRISC dimer.
(C) Integration of RAP80 into BRCA1-A results in a substantial conformation change of BRE as evidenced by a comparison of the structures of BRCA1-A and BRISC.