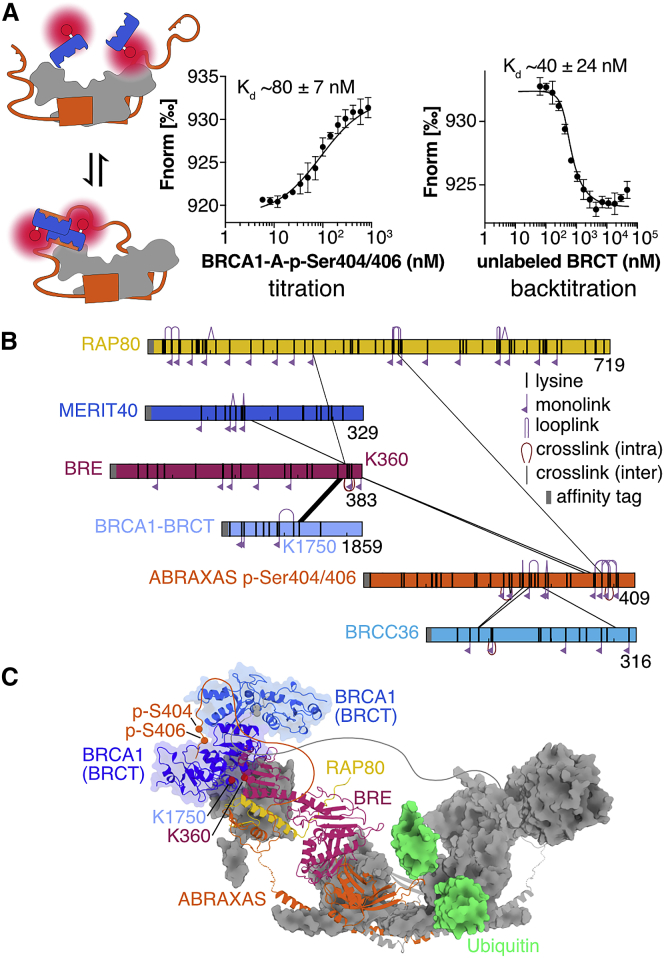
Figure 4.
BRCA1-A Forms a Defined High-Affinity Complex with BRCA1
(A) In an MST assay measuring binding of labeled BRCA1-BRCT to full-length BRCA1-A complex including phosphorylated p-Ser404/406 ABRAXAS C terminus, BRCA1-BRCT is bound with nanomolar affinity. The assay measures change of relative fluorescence during heating; values are Fnorm = Fhot / Fcold. Error bars represent mean ± SD of n = 4 replicates. Back titration with unlabeled BRCA1-BRCT confirms nanomolar affinity. Error bars represent mean ± SD of n = 4 replicates.
(B) Crosslinking network of the BRCA1-A-BRCA1 complex. Proteins are shown schematically as bars. Crosslinks are shown as black lines. The crosslink between K360 of BRE and K1750 of BRCA1 is conditional on the presence of p-Ser404/406 phosphorylation on ABRAXAS C terminus.
(C) Model of the BRCA1-A-BRCA1 high-affinity complex. One protomer of ABRAXAS, BRE, and RAP80 is shown as cartoon, while the remainder of the BRCA1-A complex is shown as gray surface. A BRCA1-BRCT dimer (blue, cartoon) is depicted in a position that localizes K1750 of BRCA1 proximal to K360 of BRE. The unstructured C-terminal regions of ABRAXAS are depicted schematically as orange and gray lines. A di-ubiquitin (green, surface) is shown modeled into the active site.