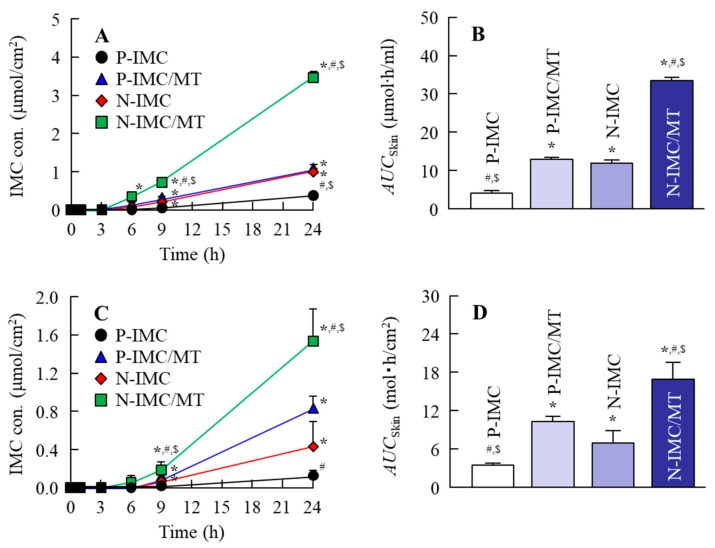
Figure 5.
In vitro skin penetration of IMC transdermal formulations. (A and B); penetration (A) and AUCSkin (B) for the IMC transdermal formulations through rat skin under normal conditions (37 °C). (C and D); penetration (C) and AUCSkin (D) for the IMC transdermal formulations through rat skin under low temperature conditions (4 °C). n = 6–8. * p < 0.05 vs. P-IMC gel for each category. # p < 0.05 versus P-IMC/MT gel for each category. $ p < 0.05 vs. N-IMC gel for each category. The combination with l-menthol enhanced the skin penetration of IMC from both the microparticles-and SNPs-based transdermal formulations, and the AUCskin values for the N-IMC and N-IMC/MT gels were 2.9 and 2.6-fold higher in comparison with the corresponding microparticles-based transdermal formulations, respectively. Although skin penetration from the P-IMC/MT gel did not differ significantly between 4 °C and 37 °C, the skin penetration from the N-IMC/MT gel was remarkably attenuated at 4 °C.