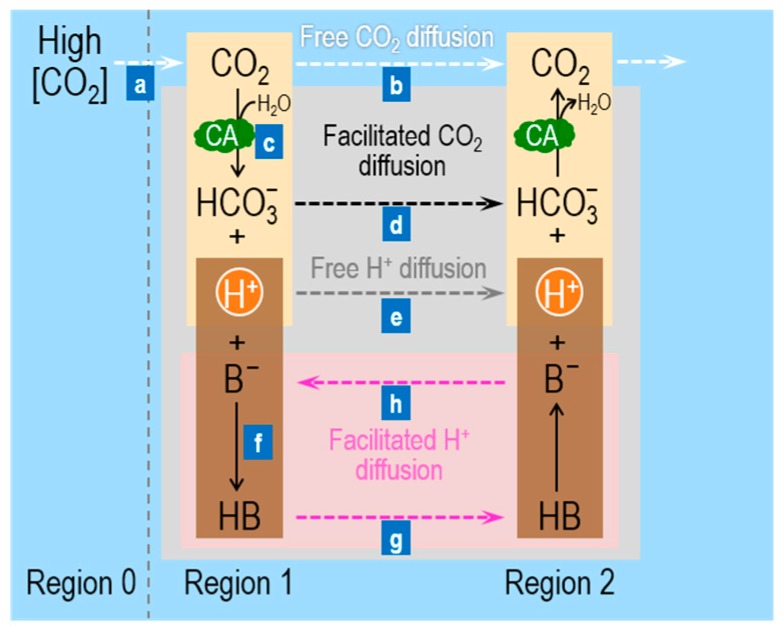
Figure 1.
Schematic of the reaction and diffusion processes involved in facilitated CO2 diffusion. Before time zero, we assume that Regions 0, 1, and 2 have identical compositions, with all reactions being in equilibrium and no concentration gradients existing between Regions. At time zero, we establish a CO2 gradient from Region 0 (high [CO2]) to Region 1 (low [CO2]) in the presence of HB/B−, a generic non-CO2/HCO3− buffer. Although CO2 is the only species moving from Region 0 to Region 1, all species can move between Region 1 and Region 2. Some CO2 moves from Region 1 to Region 2 by free diffusion. The shaded grey area identifies facilitated CO2 diffusion (i.e., HCO3− and H+ moving in the same direction as CO2) from Region 1 to Region 2. The pink shaded area identifies facilitated H+ diffusion (i.e., the antiparallel movements of HB and B−, with HB [the weak acid] moving in the same direction as CO2 [the potential weak acid]). The solid arrows identify reactions, and the dashed arrows identify free solute diffusion. These reaction and diffusion events greatly accelerate the transfer of carbon (in the guise of CO2 or HCO3−) from Region 1 to Region 2. For details, see text. CA, carbonic anhydrase.