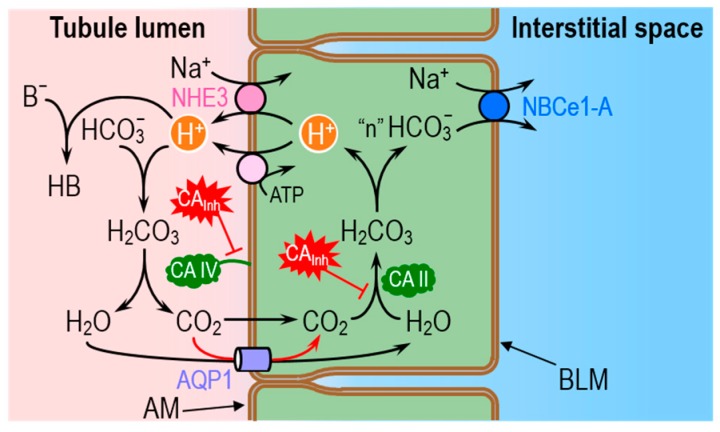
Figure 7.
Classical model of HCO3− reabsorption and titration of non-CO2/HCO3− luminal weak bases by the renal proximal tubule (PT). According to the classical view, the only acid–base transporters at the apical membrane (i.e., the membrane facing the tubule lumen) are the Na-H exchanger NHE3 and the vacuolar-type H+ pump. The energy for Na-H exchange comes from the inward Na+ gradient, established by the Na-K pump on the basolateral membrane (not shown), and the energy for the apical H+ pump comes from ATP hydrolysis. HCO3− reabsorption (or reclamation) occurs when the H+ secreted in the tubule lumen rapidly combines with HCO3− (previously filtered in the glomerulus) to form H2CO3, which slowly dissociates to form H2O and CO2. Although the figure shows the GPI-linked enzyme carbonic anhydrase (CA) IV as catalyzing the slow dehydration of H2CO3, in fact CA IV bypasses this slow step by catalyzing the direct conversion of HCO3− and H+ to H2O and CO2. The CO2 and H2O newly formed in the lumen then diffuse into the PT cytosol, mostly through the channel aquaporin AQP1. In the cytosol, although the figure shows cytosolic CA II catalyzing the slow hydration of CO2 to form H2CO3, in fact, the CA II directly converts CO2 and H2O to H+ and HCO3−. The H+ recycles back into the lumen. The HCO3− exits the PT cell across the basolateral (i.e., blood-side) membrane via the electrogenic Na/HCO3 cotransporter, which exports the equivalent of 2 or 3 HCO3− ions with 1 Na+ ion. The process just described merely reclaims previously filtered HCO3−; it does not titrate any acids in the body. A second fate of the H+ secreted in the lumen can be its combination with luminal NH3—which the PT cell generates from glutamine and glutamate—to form NH4+, most of which appears in the urine. This process is termed ammonium secretion. A third fate of the secreted H+ is to titrate weak bases other than HCO3− or NH3 (e.g., phosphate, creatinine) to form the conjugate weak acid. The amount of such acid is termed the titratable acidity. In the figure, we show all of these non-HCO3− titrations as the idealized reaction B− + H+ → HB. Of course, B can have any valence (e.g., 0 in the case of NH3), and HB has a valence 1 greater than that of B (+1 in the case of NH4+). A fourth and final fate of the secreted H+ is to remain unbuffered and thereby lower the pH of the tubule fluid. Whether the secreted H+ titrates NH3 or another non-CO2/HCO3− buffer, or remains unbuffered, one “new HCO3−” moves via NBCe1-A into the interstitial space to titrate acids throughout the body. AM, apical membrane; BLM, basolateral membrane; CAInh, carbonic anhydrase inhibitor.