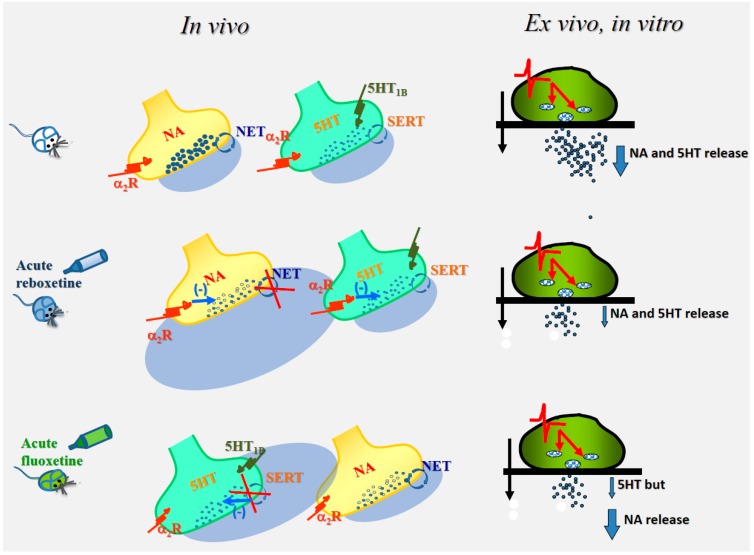
Figure 1.
The cartoon describes the mechanisms proposed to trigger the “ex-vivo, in vitro” modifications of noradrenaline and serotonin exocytosis (blue arrow) in hippocampal synaptosomes isolated from animals acutely treated with reboxetine (middle) or fluoxetine (bottom) when compared to control condition (untreated animals, top). Noradrenergic terminals possess α2 autoreceptors, while serotonergic receptors possess both 5HT1B autoreceptors and α2 heteroreceptors. It is proposed that the “in vivo” activation of both the α2 autoreceptors and the α2 heteroreceptors that would follow the acute administration of reboxetine might account for the reduced exocytosis of both noradrenaline and serotonin, while, based on notion that noradrenergic terminals do not possess serotonergic release-regulating heteroreceptors (left side of the Figure), serotonin-induced activation of serotonergic receptors in fluoxetine-treated animals only can occur at serotonergic terminals. The functional adaptations elicited by the “in vivo” activation of the presynaptic release-regulating receptors are retained by isolated synaptosomes and emerge subsequently in “in vitro” studies as changes in release efficiency (right side of the panel). Reboxetine and fluoxetine block the NA transporter (NET) and the 5HT transporter (SERT, circular arrow), increasing NA and 5HT bioavailability in the synaptic cleft. Synaptosomes were labelled with radioactive tracers and superfused with superfusion medium (black arrow). Synaptosomes were transiently (90 s) exposed to a depolarizing stimulus (the red line indicating the change in membrane potential) that causes vesicular exocytosis (red arrow) of transmitters (black dots).