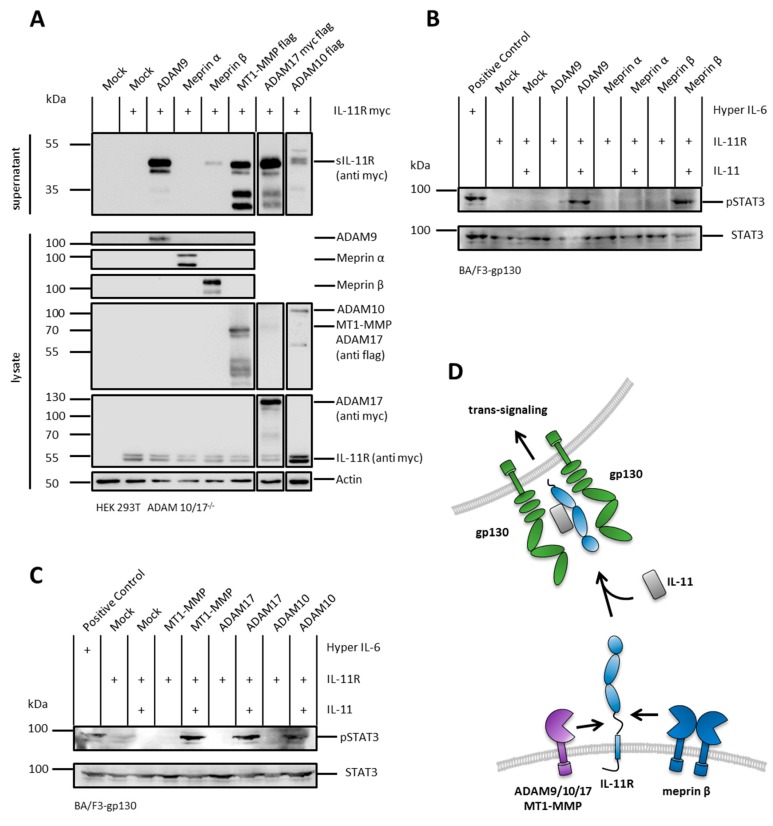
Figure 1.
The membrane bound interleukin-11 receptor (IL-11R) is shed by different metalloproteases and induces interleukin-11 (IL-11) trans-signaling. (A) Shedding of the IL-11R in HEK293T ADAM 10/17−/− cells. Cells were transfected with a N-terminally myc-tagged variant of the IL-11R alone or with ADAM9, meprin α, meprin β, membrane-type 1 matrix metalloprotease (MT1-MMP), ADAM17, or ADAM10. Supernatants were ultracentrifuged, trichloroacetic acid (TCA)-precipitated, and analyzed by immunoblotting with an antibody raised against the myc-tag. ADAM9, meprin α, and meprin β were detected with specific antibodies in lysate controls. MT1-MMP was flag-tagged, IL-11R was myc-tagged, and ADAM17 flag-/myc-tagged. pcDNA3.1 served as mock and actin as loading control. Cotransfection of IL-11R and ADAM10 was performed in an independent experiment. (B) Phosphorylation of STAT3 in Ba/F3-gp130 cells stably transfected with gp130. Cells were treated with supernatants from the experiments in (A) and with recombinant IL-11. Phosphorylation of STAT3 was analyzed for cotransfection experiments of IL-11R with ADAM9, meprin α, and meprin β. A fusion protein consisting of soluble IL-6R and IL-6 (Hyper IL-6) served as positive control for pSTAT3. Mock control from (A) served as negative control. Phosphorylation was detected with an antibody raised against phosphorylated STAT3. Total STAT3 protein served as loading control. (C) Same experimental setting as in (B) but for MT1-MMP, ADAM17, and ADAM10 as IL-11R sheddases. (D) Schematic overview of IL-11R shedding by ADAM9, ADAM10, ADAM17, MT1-MMP, and meprin β.