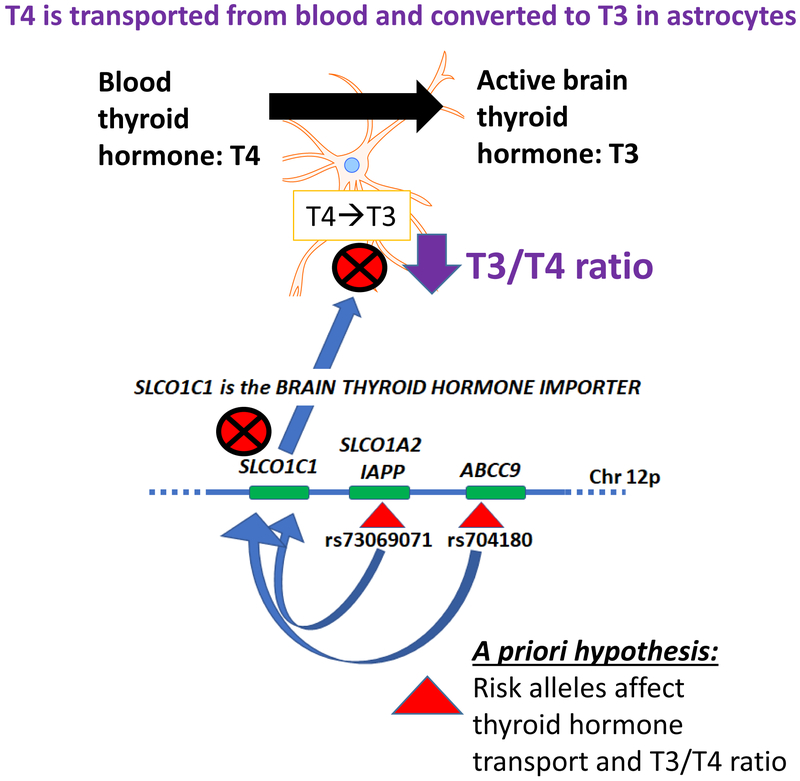
Figure 1. Schematic representation of a priori hypothesis.
An underlying hypothesis for this study is that HS-Aging linked gene variants on Chr. 12p may affect the level or activity of SLCO1C1, which is the primary brain thyroid hormone importer protein (Heuer and Visser, 2009). Rationale for this hypothesis was presented in a prior study (Nelson et al., 2016a) and the direct relevance of thyroid hormone to the brain disease was supported by data in a community-based cohort in which thyroid hormone dysregulation was found to be a risk factor for hippocampal sclerosis of aging (Trieu et al., 2018). Astrocytes import and process T4 (thyroxine) from blood, convert T4 into T3 (triiodothyronine), and then deliver T3 to neurons. A specific prediction of the study is that if SLCO1C1 functions to import T4 from blood into astrocytes for conversion into the active T3 molecules, then, a perturbation of SLCO1C1 may affect the ratio of T3 to T4 in brain parenchyma. Further, that ratio could be associated with altered risk for hippocampal sclerosis and/or TDP-43 pathology.