Fig. 2.
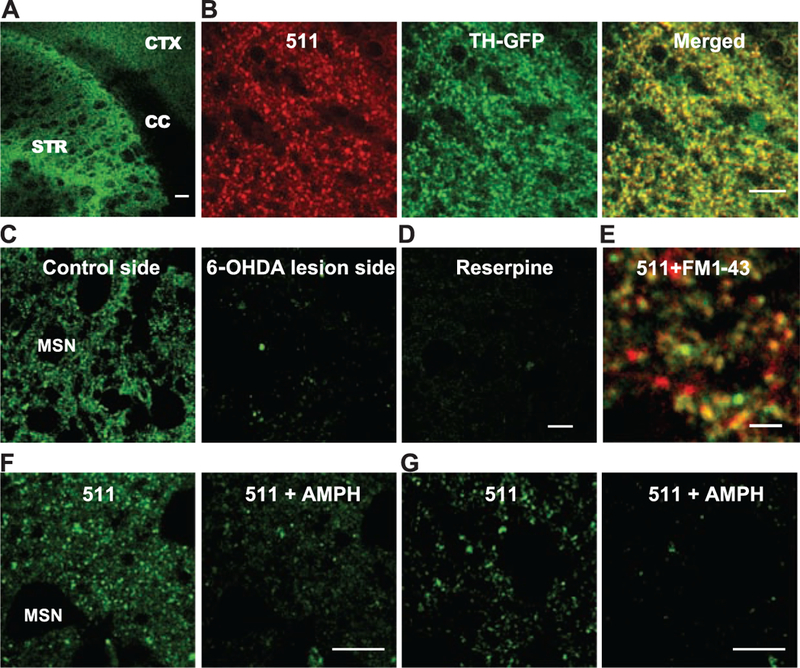
FFN511 labels dopamine terminals in live cortical-striatal acute slices. (A) Labeling by FFN511 in acute live cortical-striatal slice: abundant labeling in the striatum (STR), sparser labeling in cortex (CTX), and no label in corpus callosum (CC). Scale bar, 100 μm. (B) Overall pattern of FFN511 (red) and TH-GFP (green) fluorescent markers shows extensive overlap (yellow) as expected for dopamine terminals. The GFP-label (green) is cytosolic and thus fills both terminals and axons, whereas FFN511 labels terminals and is more punctate. Scale bar, 10 μm. (C) Nearly complete inhibition of striatal FFN511 labeling 21 days after unilateral 6-OHDA lesion. Striatal slices were examined by means of cyclic voltammetry to ensure the complete loss of dopamine release in the lesion side. (D) FFN511 labeling was strongly inhibited by reserpine (20 μM). Scale bar for C and D, 10 μm. (E) Colocalization of FM1–43–(red) and FFN511-labeled (green) terminals. Slices were sequentially loaded with 10 μM FM1–43 and 5 μM FFN511. A representative image from four independent experiments depicts extensive colocalization of FFN511- and FM1–43–labeled terminals. Scale bar, 4 μm. (F and G) Destaining of FFN511 from the striatum (F) and mPFC (G) by amphetamine. (Left) Before amphetamine; (Right) after 20 min of 20 μM amphetamine. Scale bar, 10 μm.
