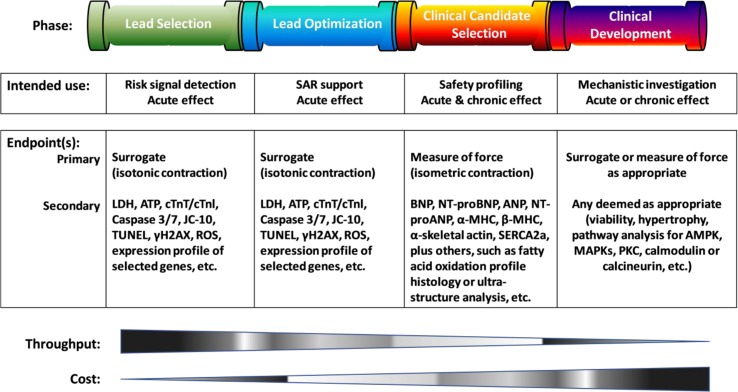
Figure 2.
A schematic overview of endpoints proposed to use at different phases of drug discovery and development, along with the required throughput and associated cost. The primary endpoint refers to functional measurements to quantify contractile force generation indirectly with a surrogate (often employing optical technique to detect shortening—namely, isotonic contraction of cardiomyocytes beating freely without a load) or directly in the presence of both pre- and after-loads. The secondary endpoint is used to detect cell injury, potentials, or underlying mechanisms for long-term modeling. SAR, structure-activity relationship; LDH, lactate dehydrogenase; ATP, adenosine triphosphate; cTnT/cTnI, cardiac troponin T/I; TUNEL, terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling; γH2AX, gamma H2A histone family member X; ROS, reactive oxygen species; BNP, B-type natriuretic peptide; ANP, atrial natriuretic peptide; NT-proBNP or proANP, N-terminal pro B-type or atrial natriuretic peptide; α-/β-MHC, alpha- or beta-myosin heavy chain; SERCA2a, sarcoplasmic reticulum Ca2+ ATPase; AMPK, 5’AMP-activated protein kinase; MAPKs, mitogen-activated protein kinase; PKC, protein kinase C.