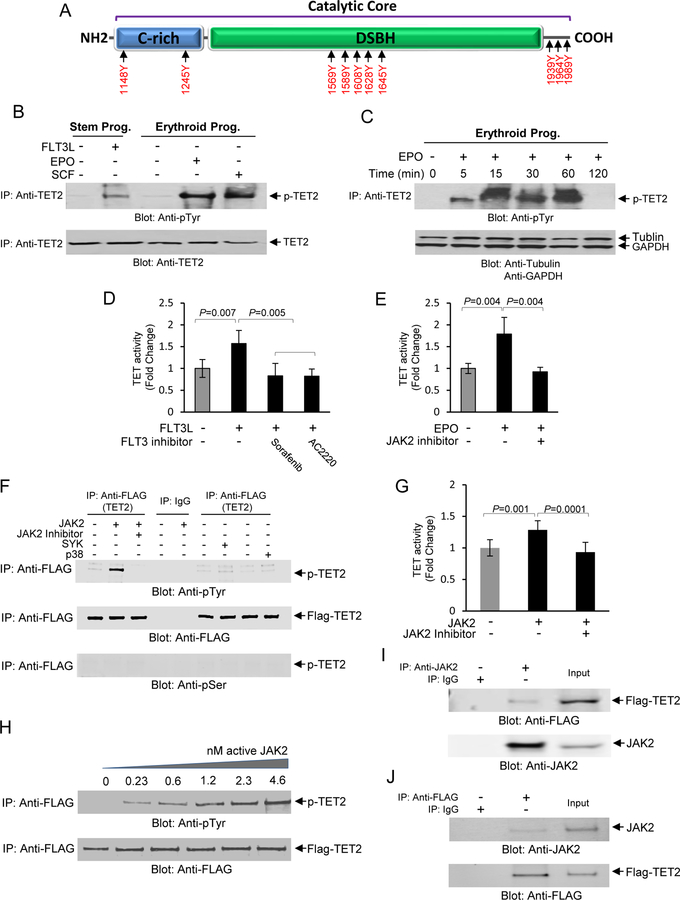
Fig. 2. Phosphorylation of TET2 by JAK2 in response to hematopoietic cytokines.
(A) In-Silico analysis using KinasePhos 2.0 and PPSP computational program for prediction of sites for tyrosine phosphorylation within the catalytic core of TET2 protein. The cysteine-rich (C-rich) and DSBH domains are depicted as well as the C-terminal region. (B) Total cell lysates were collected from CD34+ stem/early progenitor cells or from erythroid progenitors after stimulation with FLT3L, SCF or EPO as indicated and subjected to immunoprecipitation using an anti-TET2 antibody followed by immunoblot analysis using an anti-phospho-tyrosine antibody. The same blot was then used for immunoblotting against an anti-TET2 antibody as a protein loading control. (C) A time course determination of TET2 phosphorylation in response to EPO (10U/ml) in primary erythroid progenitors. The same blot was then used for immunoblot analysis using tubulin and GAPDH as protein loading controls. (D) Inhibition of TET2 activity by FLT3 inhibitors, Sorafenib (10 nM), and AC2220 (10 nM), in CD34+ stem/early progenitors. Cells were exposed to the inhibitors for 1 hr prior to stimulation with FLT3L for 30 minutes followed by determination of TET activity. Cytokine unstimulated and FLT3L stimulated samples without the presence of inhibitors were used as controls. Data are represented as mean SEM ± from three biological replicates. (E) Inhibition of TET2 activity by JAK2 Inhibitor Ruxolitinib (100 nM) in primary erythroid progenitors. Cells were exposed to the inhibitor for 1 hour prior to stimulation with EPO for 30 minutes followed by determination of TET activity. Unstimulated and EPO stimulated samples without the inhibitor was used as controls. Data are represented as mean SEM ± from three biological replicates. (F) An in vitro kinase assay was developed using recombinant GFP/Flag-tagged TET2 catalytic domain and active JAK2 kinase under cell-free conditions for ascertaining whether TET2 catalytic domain is a direct target for phosphorylation by JAK2. In vitro kinase assays were performed using the GFP/Flag-tagged catalytic domain (1129–2002) of TET2 together with recombinant active JAK2, SYK and p38 MAP kinases as indicated. In vitro kinase reaction products were resolved on a SDS-PAGE gel and immunoblot analysis performed using an anti-phospho-tyrosine antibody. As controls the immune blots were also exposed to anti-FLAG and anti-phospho-serine antibodies to confirm immunoprecipitation reactions in all experimental samples were intact and phosphorylation was tyrosine specific respectively. (G) The in vitro kinase reaction products were analyzed for TET activity in the presence or absence of the JAK2 inhibitor by ELISA. Data are represented as mean SEM ± from three biological replicates. (H) Phosphorylation of TET2 by JAK2 is dose dependent. In vitro kinase assays were performed using various concentrations of recombinant JAK2 followed by analysis of the reaction products by immunoblot analysis using an anti-phospho-tyrosine antibody followed by immunoblot analysis using an anti-FLAG antibody as a protein loading control. (I) TET2 specifically interacts with JAK2 in UT-7 erythroid cells. Total cell lysates from UT-7 erythroid cells transfected with GFP/Flag-tagged TET2 catalytic domain were used to immunoprecipitate JAK2 using and anti-JAK2 antibody and the immunoprecipitates were subjected to immunoblot analysis using an anti-FLAG antibody. As a control the same lysates were subjected to immunoblot analysis using an anti-JAK2 antibody. (J) A reciprocal immunoprecipitation coupled immunoblot analysis was performed using anti-FLAG antibody for immunoprecipitation followed by immunoblot analysis using an anti-JAK2 antibody. As a control the same lysates were subjected to immunoblot analysis using an anti-FLAG antibody.