Figure 1:
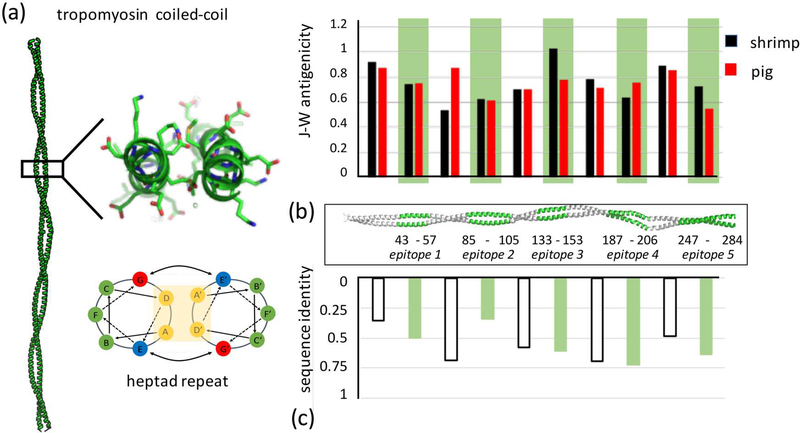
(a) Tropomyosin forms an extended parallel coiled-coil dimer that consists of approximately 40 uninterrupted heptad repeats, a regular pattern of seven residues whose position can be described as a to g based on their position along the helix. Structure shown is pig Tpm - PDB ID 1C1G (Whitby and Phillips, 2000). (b) The five major epitopes identified in previous studies (Ayuso et al., 2002) are highlighted in green along the coiled coil. The J-W index computes antigenicity based on sequence. For shrimp Tpm, there is no statistical difference between epitope and non-epitope antigenicities (student’s t-test p = 0.86), or between shrimp and pig epitope regions (p = 0.50). (c) Cross-reactivity has not been observed between shrimp and pig Tpms. This is likely not due to lower sequence identity within epitopes versus non-epitope regions (p = 0.99).