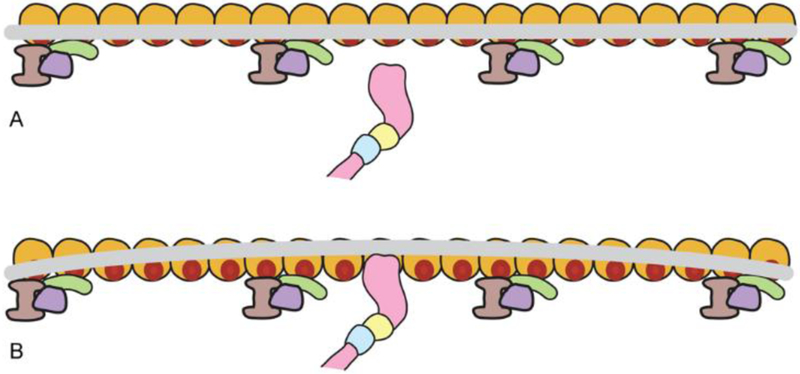
Figure 2. Tropomyosin response to myosin binding along F-actin.
Schematic representation of a single myosin head (pink) binding to an actin target site (red on gold actin subunit) along one chain of F-actin, moving the tropomyosin cable (grey) locally from the blocked (or closed) state position (panel A) to an M-state location (panel B)(single actin-tropomyosin strands shown for simplicity). Given tropomyosin’s stiffness, stretches of the tropomyosin cable close to the displacement will also move in tandem and expose additional myosin target sites on neighboring actin subunits (panel B). However, because tropomyosin is not a completely rigid rod, correlation between the initial displacement and any corresponding shift further along the coiled coil will diminish with distance. For example, at a point 40 nm from the myosin binding, tropomyosin’s position still will correlate with the initial displacement but, at this location, fluctuate back and forth by about ± 25° (see Fig. 3). The propagation of the myosin-induced repositioning will also be opposed by outlying troponin complexes (lavender, purple, green).