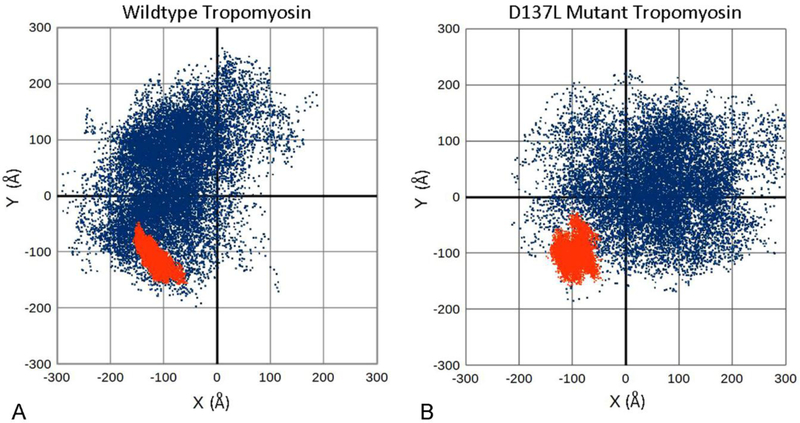
Figure 4. Anisotropic bending of tropomyosin during MD.
A, B. x, y coordinates of the C-terminal positions of tropomyosin conformers during MD, plotted following alignment of the first 28 N-terminal residues along the z-axis. Here, each point represents the C-terminal position of an individual conformer taken during MD, projected onto a plane perpendicular to the z-axis of tropomyosin. The C-terminus of a perfectly straight, inflexible rod would plot to the 0,0 coordinate position. In contrast, a straight flexible rod displaying isotropic uniform bending would plot as a symmetrical circle of dots around the 0,0 coordinate position. A flexible rod displaying anisotropic, preferential bending would be biased to one or another x, y quadrant and plot accordingly. A. During MD, the motions of isolated wildtype tropomyosin (blue dots) are anisotropic, reflecting an out-of-plane bending bias of the coiled coil. The motions of actin-associated tropomyosin (red dots) are more limited in direction. Here, both the magnitude and the direction (largely azimuthally and in plane) of the fluctuations are damped out by actin interaction. B. Motions of isolated D137L mutant tropomyosin (blue dots) are considerably less anisotropic, reflecting the relatively straight tropomyosin conformation, and consistent with the increased global flexibility of the mutant. The motions of actin-associated D137 tropomyosin (red dots) now show biphasic anisotropy as the mutant adapts to the F-actin helix during MD while clinging to the actin surface. Data presented calculated from coordinates in Li et al (2010, 2011) and Lehman et al (2018) and from new MD studies on actin-D137L tropomyosin (performed as previously) (Li et al 2011; Lehman et al 2018).