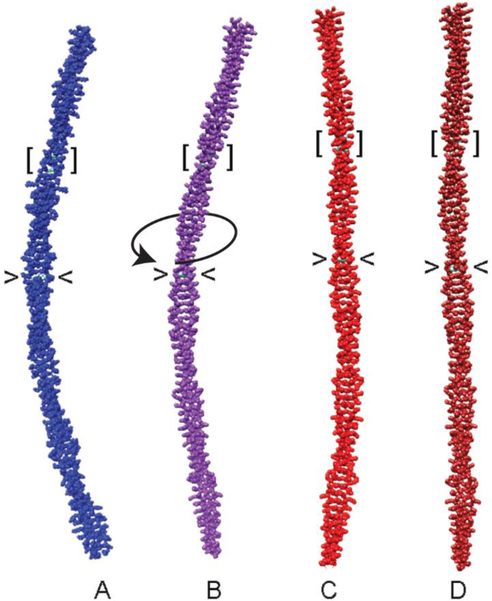
Figure 5. Mutation straightens tropomyosin.
(A) Canonical model of tropomyosin with idealized coiled-coil symmetry (Lorenz et al 1995). Average structures during MD simulations of (B) wildtype tropomyosin, (C) A74L-A78V-A81L, and (D) D137L mutant tropomyosins. The position of residues 137 (angle brackets) and 74, 78 and 81 (square brackets) are indicated. Over-twisting of tropomyosin initiated near to residue 137 is indicated by a curved arrow in panel B. Note the gentle bend of the wildtype coiled coil (B) and the straightening of the mutant coiled coils (C, D). Also note that localized substitution of critical residues has delocalized global effects on tropomyosin structure. Figure generated from coordinates in Li et al (2010) and Moore et al (2011).