Abstract
Introduction:
Optimal endpoints in phase II trials evaluating salvage therapy for metastatic urothelial carcinoma (mUC) are necessary to identify promising drugs, particularly immunotherapeutics, where response and progression-free survival may be unreliable. We developed a nomogram using data from phase II trials of historical agents to estimate the 12-month overall survival (OS) for patients to which observed survival of nonrandomized datasets receiving immunotherapies could be compared.
Materials and methods:
Survival and data for major prognostic factors were obtained from phase II trials: hemoglobin, performance status, liver metastasis, treatment-free interval and albumin. A nomogram was developed to estimate 12-month OS. Patients were randomly allotted to discovery:validation (DIS:VAL) datasets in a 2:1 ratio. Calibration plots were constructed in the VAL dataset and data bootstrapped to assess performance. The nomogram was tested on external nonrandomized cohorts of patients receiving pemetrexed and atezolizumab.
Results:
Data were available from 340 patients receiving sunitinib, everolimus, docetaxel + vandetanib, docetaxel + placebo, pazopanib, paclitaxel, or docetaxel. Calibration and prognostic ability was acceptable (c-index = 0.634, 95% CI = 0.596–0.652). Observed 12-month survival for patients on pemetrexed (n = 127, 23.5% [95% CI: 16.2%−31.7%]) was similar to nomogram-predicted survival (19% [95% CI: 16.5–21.5], P> 0.05), while observed result with atezolizumab (n = 403, 39.0% [95% CI: 34.1–43.9]) exceeded predicted result (24.6% [95% CI: 23.4–25.8], P< 0.001).
Conclusions:
This nomogram may be a useful tool to interpret results of nonrandomized phase II trials of salvage therapy for mUC by assessing the OS contributions of drug intervention independent of prognostic variables.
Keywords: Urothelial carcinoma, Salvage therapy, Nomogram, Survival, Atezolizumab
Microabstract
Response and progression-free survival are unreliable in providing signals of benefit of new agents especially immunotherapy in nonrandomized phase II trials of salvage therapy for metastatic urothelial carcinoma. A nomogram employing baseline prognostic variables was developed to estimate the 12-month survival of patients receiving salvage chemotherapy to which observed survival of nonrandomized datasets could be compared to interpret results.
Introduction
Taxanes have been historically used for post-platinum salvage therapy in the United States, but yield dismal outcomes, with responses in ~10% of patients and median progression-free survival (PFS) and overall survival (OS) of 2–3 months and 5–8 months, respectively 1, 2. Vinflunine was approved in other countries based on an extension of survival compared to best supportive care in the trial-eligible population 3. Outcomes with vinflunine appear similar to taxanes, with the caveat of comparison across trials 3.
Multiple agents have been evaluated for improving outcomes of mUC patients in the salvage setting, mostly in nonrandomized phase II trials4–7. More recently, the programmed death (PD)-1 and PD-ligand (L)-1 inhibitors have been investigated mostly in nonrandomized phase II trials. Indeed, atezolizumab, nivolumab, durvalumab and avelumab have been granted accelerated approval by the U.S. FDA based on durable response rates of 15–20% observed in nonrandomized phase II trials, and full approval has been granted to pembrolizumab based on survival benefit versus chemotherapy in a phase III trial 8–12.
Evaluating the clinical benefit of PD-1 and PD-L1 inhibitors in the context of nonrandomized phase II trials is challenging. The early readouts of clinical benefit seen with chemotherapy, i.e., response rates (RR), PFS, and median survival have not proven to be accurately predictive of long-term survival. Moreover, patients receiving salvage therapy exhibit a spectrum of outcomes influenced by pre-treatment prognostic factors. Thus, it is critical in the interpretation of non-comparator trials that favorable long-term outcomes reported in such studies is a function of the drug intervention and not solely due to patients with more favorable prognostic features.
We hypothesized that the ability to predict survival at a landmark timepoint beyond the usual median OS may capture delayed benefits and assist in interpreting long-term survival outcomes observed in nonrandomized phase II trials of new agents, especially immunotherapy. Previously, we identified and validated a 5-factor model which improved the prognostication of patients receiving salvage therapy with taxanes and other historically evaluated agents with similar activity 13. We sought to improve the utility of these prognostic factors by constructing a nomogram utilizing these factors to predict 12-month overall survival of a cohort of patients receiving taxanes and other historical salvage agents studied in phase 2 trials, all of which had similar outcomes. We then applied this nomogram to external datasets for further validation of the model and to assess the benefit from pemetrexed or atezolizumab compared to nomogram-predicted survival.
Patients and Methods
Patient selection
Prospective phase II trials of salvage systemic chemotherapy and/or biologic agent therapy (not including PD1/PD-L1 inhibitors) employed in our previous analyses to construct prognostic models for patients following platinum-based chemotherapy for mUC were pooled. All of these trials required previous pathological confirmation of UC and progressive disease with measurable lesions. Trials that administered taxanes or other agents considered to have modest activity similar to taxanes were selected. Trials were also selected based on the availability of individual patient level data and the ability of the respective investigators to provide these data. Data regarding survival and the 5 previously validated prognostic factors were required and collected: albumin, treatment-free interval (TFI), hemoglobin (Hb), Eastern Cooperative Oncology Group (ECOG)-performance status (PS) and liver metastasis (LM) status. The data were de-identified and these trials were approved by the Institutional Review Boards (IRBs) of the respective institutions.
Construction of validated nomogram
The patients were randomly (2:1) split into discovery and validation datasets. A nomogram using the 5 clinical prognostic factors was developed to estimate 12-month survival in the discovery dataset and externally validated in the validation dataset. 95% Bias-corrected and accelerated (BCa) confidence intervals were constructed using 2000 bootstrap samples. Calibration and prognostic ability was assessed comparing estimated versus observed 12-month OS. Concordance was measured using the concordance index. Analyses were performed in SAS v9.0 or R v.3.2.2.
Application of nomogram to external datasets
The nomogram was then applied to external nonrandomized salvage therapy datasets: 1) a single-center retrospective cohort of patients receiving pemetrexed and 2) another dataset composed of a combination of 2 single-arm trials of atezolizumab (PCD4989g and IMvigor210) 8, 12, 14, 15. Expected 12-month survival was calculated based on the nomogram for each patient and the observed 12-month survival was estimated using the Kaplan-Meier method. The difference between the observed and expected 12-month observed survival was then compared across bootstrap samples using a paired t-test.
Results
Patient characteristics
Data were available from 340 patients receiving docetaxel + vandetanib or placebo (n = 109), sunitinib (n = 77), everolimus (n = 45), pazopanib (n = 42), paclitaxel (n = 36) and docetaxel (n = 31)4, 6, 16–19. The discovery dataset consisted of 227 patients and the validation dataset included 113 patients (Table 1).
Table 1.
Patient characteristics
| Development Dataset4, 6, 16–19 |
Validation Dataset4, 6, 16–19 |
Pemetrexed Dataset 14 |
PCD4989g (atezolizumab) 8, 15 |
IMvigor210 (atezolizumab) 12 |
|
|---|---|---|---|---|---|
| N | 227 | 113 | 127 | 93 | 310 |
| N (%) Male | 140 (61.7) | 67 (59.3) | 96 (75.6) | 71 (76.3) | 241 (77.7) |
| Mean (sd) age | 62.8 (10.3) | 64.7 (10.0) | 65.3 (9.8) | 65.3 (9.1) | 65.6 (10.1) |
| N (%) ECOG-PS ≥1 | 129 (56.8) | 65 (57.5) | 108 (85.0) | 58 (62.4) | 193 (62.3) |
| N (%) Liver Metastases | 85 (37.4) | 43 (38.1) | 41 (32.3) | 34 (36.6) | 96 (31.0) |
| N (%) Hb <10 mg/dL | 33 (14.5) | 20 (17.7) | 31 (24.4) | 18 (19.4) | 69 (22.3) |
| Median (IQR) TFI from last prior therapy | 3.8 (1.6, 7.4) | 4.1 (1.6, 8.4) | 2.6 (1.2, 5.8) | 3.9 (2.1, 7.7) | 4.1 (2.1, 8.9) |
| N (%) TFI < 3 months | 94 (41.4) | 45 (39.8) | 68 (53.5) | 39 (41.9) | 121 (39.0) |
| N (%) Albumin <LLN (35) | 24 (10.6) | 14 (12.4) | 37 (29.1) | 20 (21.5) | 98 (31.6) |
| N (%) Deaths | 192 (84.6) | 100 (88.5) | 107 (84.3) | 58 (61.1) | 218 (70.3) |
ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; Hb: hemoglobin; TFI: treatment free interval; LLN: lower limit of normal; OS: overall survival
Construction and performance of nomogram
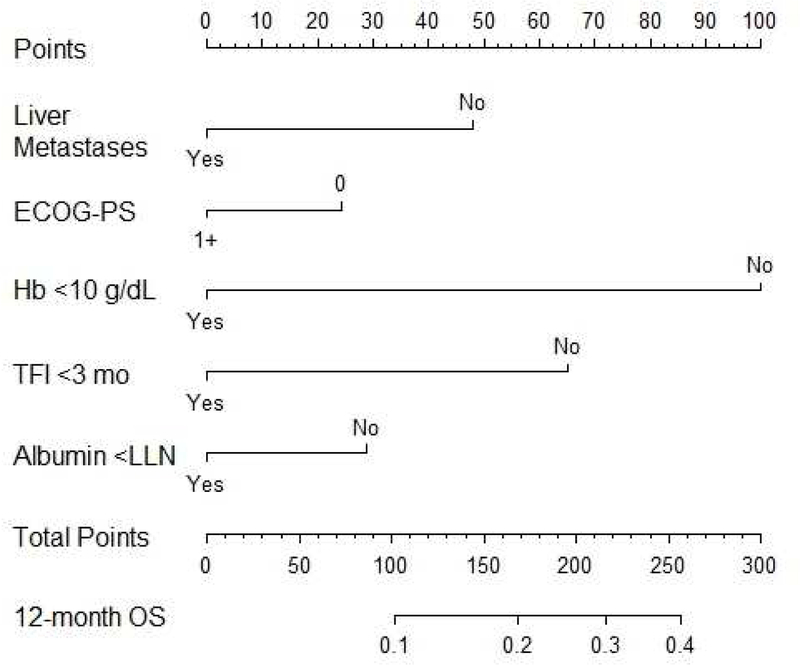
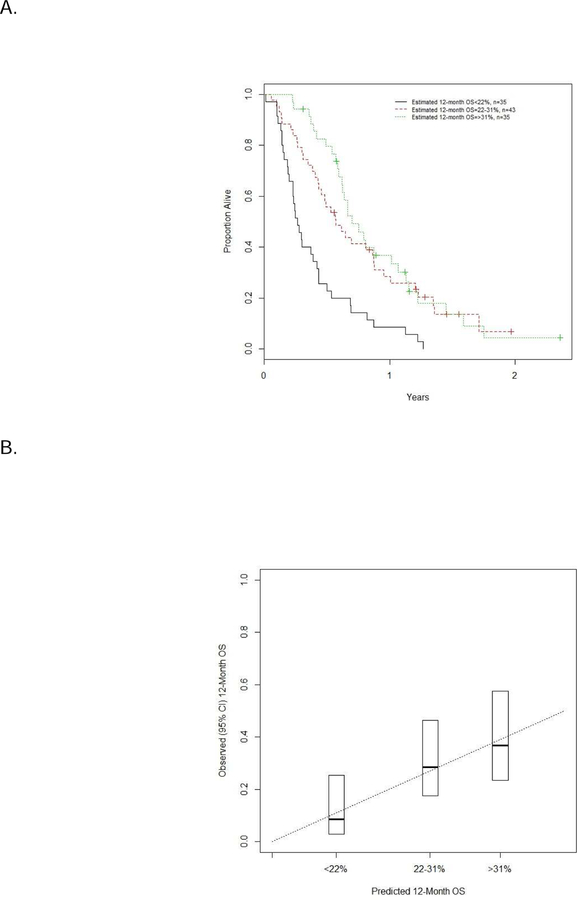
The nomogram consisting of the 5 prognostic factors was constructed from the discovery or development dataset (Figure 1). The performance of the nomogram was assessed in the validation dataset (Table 2). The estimated 12-month survival was segregated into tertiles, and estimated vs. observed 12-month survivals were compared in the validation dataset (Figure 2). Calibration and prognostic ability of the model was acceptable in the validation cohort (c-index = 0.634, 95% CI = 0.596–0.652). Additionally, the c-index was applied to the separate pemetrexed dataset since this agent demonstrates similar outcomes as agents included in the primary discovery and validation datasets above, and the c-index was 0.634 (95% CI=0.548–0.642).
Figure 1.
Nomogram to estimate 12 month survival based on baseline prognostic factors
ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; Hb: hemoglobin; TFI: treatment free interval; LLN: lower limit of normal; OS: overall survival
Table 2.
Observed and nomogram-estimated 12-month survival
| Development Dataset |
Validation Dataset |
Pemetrexed Dataset |
PCD4989g (atezolizumab) |
IMvigor210 (atezolizumab) |
|
|---|---|---|---|---|---|
| N | 227 | 113 | 127 | 93 | 310 |
| Expected 12, mo OS (95% CI) | 24.5 (22.5, 25.5) | 23.5 (20.4, 24.7) | 18.5 (15.5, 19.5) | 24.9 (22.4, 27.5) | 24.6 (23.2, 26.0) |
| Observed 12, mo OS (95% CI) | 22.2 (17.1, 28.5) | 24.9 (18.4,33.7) | 23.5 (16.1, 32.1) | 46.9 (35.7, 57.6) | 36.9 (31.6, 42.6) |
| Difference | −2.3 (−7.4, 3.5) | 1.4 (−5.6, 10.3) | 5.0 (−2.1, 13.7) | 22.1 (10.6, 33.1) | 12.3 (7.0, 18.2) |
| P-value | 0.46 | 0.74 | 0.23 | <0.001 | <0.001 |
mo: month; OS: overall survival; CI: confidence interval
Figure 2.
Tertiles of estimated 12-month survival (A) and estimated vs. observed survival (B)
The figures show the estimated 12-month survival segregated into tertiles in the validation dataset (n=113), and comparison of estimated vs. observed 12-month survivals in the validation dataset.
Use of nomogram to compare predicted vs. observed 12-month survival in external datasets
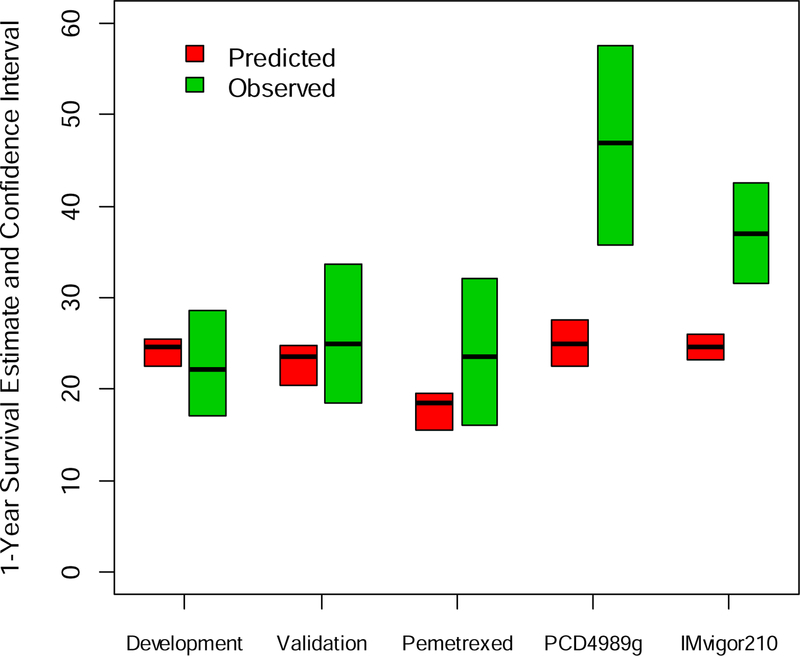
The nomogram was applied to external datasets: pemetrexed (n=127) and a dataset of patients receiving atezolizumab (n=403) composed of 2 single-arm trials (PCD4989g [n=93] and IMvigor210 [n=310]) (Table 2, Figure 3). The c-index was 0.759 for the PCD4989g dataset and 0.690 for the IMvigor210 dataset. Observed 12-month survival for patients on pemetrexed (23.5% [95% CI: 16.2%−31.7%]) was similar to nomogram-predicted survival (19% [95% CI: 16.5–21.5], P> 0.05). In contrast, the observed 12-month survival with atezolizumab (46.9% [95% CI: 35.7–57.6] for PCD4989g and 36.9% [95% CI: 31.6–42.6] for IMvigor210)) significantly exceeded predicted results (24.9% [95% CI: 22.4–27.5] for PCD4989g and 24.6% [95% CI: 23.2–26.0] for IMvigor210, P< 0.001 for both studies). The observed result with the combination of the 2 atezolizumab datasets (39.0% [95% CI: 34.1–43.9]) also exceeded the predicted result (24.6% [95% CI: 23.4–25.8], P< 0.001).
Figure 3.
Observed vs. nomogram-estimated 12-month survival of patients receiving pemetrexed or atezolizumab
The development (discovery, n=113) and validation datasets (n=227) were derived from prospective phase II trials of salvage systemic chemotherapy and/or biologic agent therapy (not including PD1/PD-L1 inhibitors). The pemetrexed dataset (n=127) was a retrospectively collected dataset of patients receiving salvage pemetrexed. PCD4989g (n=93) and IMvigor210 (n=210) were separate phase 1b and II trials, respectively, which evaluated atezolizumab as salvage therapy for mUC
Discussion
Dissecting the impact on survival with novel drugs from the impact of pre-existing prognostic factors in non-comparative trials is not possible due to patient heterogeneity and marked variability of prognostic factors across trials. Though phase 3 trials that control for known prognostic factors across arms remain the gold standard, there is a compelling need for an intermediate methodology to assess clinical benefit seen in nonrandomized phase 2 trials. Nomograms can predict individual patient outcome by incorporating a weighted contribution of each prognostic factor, and have been used to predict individualized risk estimates for 1-, 2- and 5-year survival of mUC patients treated with first-line chemotherapy 20, 21. This study has extended the nomogram methodology to the post-platinum setting by incorporating 5 baseline prognostic factors (albumin, Hb, ECOG-PS, TFI and LM), providing 12-month survival estimates and demonstrating its usefulness in identifying the survival benefit from novel drugs. Thus, the nomogram facilitates the interpretation of nonrandomized salvage therapy data and comparison of results across trials, especially in the era of immunotherapy. While trials report baseline characteristics, they frequently vary across trials and all 5 validated prognostic factors are not always reported, which render comparison of outcomes across trials problematic, while the application of our nomogram allows the rigorous comparison of observed vs. expected survival after controlling for each factor in individual patients.
This trial-specific nomogram was validated using a randomized internal cohort of patients and then applied to 2 external datasets of patients, one that received pemetrexed and another cohort of trial patients receiving atezolizumab. Atezolizumab was associated with a significantly longer 12-month survival compared to nomogram-predicted survival, while treatment with pemetrexed showed no survival difference. While the landmark 12-month survival endpoint was selected to better capture the benefits from immunotherapy, this nomogram is also applicable to interpreting nonrandomized phase II trials evaluating other cytotoxic, biologic and targeted agents.
The estimated 12-month survival rate of the atezolizumab and pemetrexed datasets if these patients had received historical agents is similar to the 12-month survival observed in the control chemotherapy arm of the Keynote-045 phase III trial that compared salvage taxane/vinflunine vs. pembrolizumab11. The 12-month survival of ~40% observed by atezolizumab has also been observed with pembrolizumab in the Keynote-045 trial and can be considered a benchmark when designing future phase II clinical trials of salvage systemic therapy for mUC. These findings reinforce the value of this nomogram to interpret outcomes in nonranzomized phase II trials of new agents. Indeed, PD-1 and PD-L1 inhibitors including nivolumab, durvalumab and avelumab have all been investigated in nonrandomized phase II trials 9, 22, 23. However, in contrast, pembrolizumab and atezolizumab, have been investigated in phase III trials in the salvage setting ( [KEYNOTE-045], [IMvigor211]). Hence, application of this nomogram to the nonrandomized phase II trials may be especially useful since phase III salvage trials of these agents are not planned.
Recently, it was reported that the Phase III IMvigor211 study that compared atezolizumab vs. chemotherapy (vinflunine or taxane) for progressive post-platinum mUC patients did not meet its primary endpoint of superior OS in atezolizumab-treated patients 24. However, the results observed with atezolizumab in IMvigor211 were consistent with those observed in the preceding Phase II IMvigor210 trial. Specifically, any evidence for imbalances between the arms for known prognostic factors needs scrutiny since the chemotherapy arm appeared to over-perform. Furthermore, this trial stratified patients for the older Bellmunt model (which does not include TFI and albumin), chemotherapy agent and PD-L1 status 25. The PD-L1 expression by immune cells using the SP142 antibody by this trial has also been demonstrated to be an outlier and exhibits greater variability compared to other assays 26. Potentially, the statistical design of the trial may have compromised the ability to identify an extension of survival. Notably, the primary endpoint, overall survival, was evaluated in a hierarchical fashion in cohorts defined by PD-L1 expression. However, the requirement for the high PD-L1 expressing population to exhibit an increment in survival may have undermined the ability to identify a benefit in the entire intention to treat population.
Thus, the IMvigor211 trial may be an optimal independent dataset for potential future analysis applying this novel nomogram methodology because the prognostic factors utilized in the nomogram are different than those used for stratification in the trial. Such an analysis may provide broader insight into whether: 1) the prognostic variables for the trial are better than those used in the nomogram methodology, or vice versa; 2) imbalances across treatment arms exist that may not have been evident using conventional stratification; 3) the conventional arm or any individual drug cohort (i.e., paclitaxel, docetaxel, or vinflunine) performed better than expected when controlled for nomogram-specific prognostic factors.; and/or 4) the investigational arm with atezolizumab underperformed due to a greater proportion of nomogram-specific poor prognostic factors.
Our study has limitations inherent to retrospective studies. Ideally, the nomogram to predict 12-month survival should have included only patients receiving taxanes or vinflunine. However, individual level data from an adequate number of patients receiving taxanes or vinflunine were not available. Hence, in addition to patients receiving single agent taxanes, the discovery and validation datasets used data from patients receiving docetaxel plus vandetanib, sunitinib, everolimus and pazopanib. Nevertheless, the dismal overall outcomes observed in phase II trials evaluating sunitinib, everolimus and pazopanib appear similar to outcomes seen with single agent taxanes or vinflunine, and it may be considered reasonable to utilize such patients to construct the nomogram. The use of data from patients who received docetaxel combined with vandetanib may be questioned, but it must be noted that this combination did not demonstrate any statistical differences in all endpoints when compared with docetaxel alone in a randomized phase II trial. Since the nomogram was constructed using data derived from patients enrolled on phase II trials, its applicability to a retrospectively assembled dataset such as the pemetrexed dataset or a phase I trial dataset (PCD4989g) may be questioned. However, the nomogram did control for 5 major baseline prognostic factors. While phase I trial patients were more heavily pretreated, we have reported earlier that the number of prior lines of therapy was not independently significant after controlling for other prognostic factors 27. The salvage chemotherapy trials employed to construct the nomogram were completed years prior to the atezolizumab trials, and the confounding impact of recently improved supportive care may be a potential limitation of comparing the two cohorts. The c-index (0.63) may be considered modest although this is similar to nomograms proposed in other settings, and molecular factors may provide a more optimal c-index. Indeed, tumor subtypes based on gene expression and tumor mutation burden may improve our ability to prognosticate12. The survival at 12 months cannot be ascertained at an early timepoint, unlike response and PFS. However, a valuable intermediate endpoint at an early timepoint that translates to extension of survival is unclear especially in the context of immunotherapy. Although durable responses are seen in 15–20% of patients, a substantial proportion of nonresponders also benefit, as suggested by an improvement in median OS without an improvement in median PFS with pembrolizumab11. Our group has previously proposed the use of a nomogram based on baseline prognostic factors to estimate PFS at 6 months to interpret nonrandomized phase II trials28. However, PFS appears suboptimal to capture benefit from immunotherapy. The application of this nomogram to the other PD1/PD-L1 inhibitor datasets in the salvage mUC setting is desirable and such efforts are underway, which will enable interpretation of results across trials and potentially identify promising novel agents. Unfortunately, pharmaceutical companies are unlikely to aim to compare their agents with competing agents manufactured by other companies.
While this nomogram may be a useful tool to interpret results of nonrandomized phase II trials of salvage therapy for mUC, it cannot replace phase III trials as definitive evidence. A novel prognostic model in the setting of salvage PD-1/PD-L1 inhibitors is necessary, since different factors may be more relevant in this setting. This would enable the construction of successive generations of nomograms to interpret survival in nonrandomized phase II trials to enable virtual comparisons. With the emergence of PD1/PD-L1 inhibitors as first-line therapy of cisplatin-ineligible patients based on nonrandomized phase II trials, nomograms developed in the first-line platinum-based chemotherapy setting may be utilized to interpret these data 21. Moreover, new nomograms will be necessary for the first-line single agent PD1/PD-L1 inhibitor setting to help interpret new regimens undergoing nonrandomized phase II evaluation in this setting29, 30.
Conclusions
A nomogram was developed incorporating 5 validated baseline prognostic factors to estimate 12-month survival in the context of patients receiving historical agents to treat progressive mUC following platinum-based chemotherapy. The application of this nomogram to nonrandomized datasets of patients receiving pemetrexed or atezolizumab suggested that atezolizumab extends survival compared to historical agents while pemetrexed does not.
Clinical practice points.
Since response and progression-free survival may be unreliable to identify promising drugs, particularly immunotherapeutics, optimal endpoints are necessary to interpret results of phase II trials evaluating salvage therapy for metastatic urothelial carcinoma (mUC).
We developed a nomogram using data using baseline prognostic factors from phase II trials of historical agents to estimate the 12-month overall survival (OS) for patients to which observed survival of nonrandomized datasets receiving immunotherapies could be compared.
This nomogram may be a useful tool to interpret results of nonrandomized phase II trials of salvage therapy for mUC by assessing the OS contributions of drug intervention independent of prognostic variables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Genitourinary Cancer Symposium, Orlando, Florida, USA
Disclosures:
Guru Sonpavde: Consultant for Bayer, Sanofi, Pfizer, Novartis, Eisai, Janssen, Amgen, Astrazeneca, Merck, Genentech, Argos, Agensys; Research support to institution from Bayer, Onyx, Celgene, Boehringer-Ingelheim, Merck, Pfizer; Author for Uptodate; Speaker for Clinical Care Options
Andrea Necchi: Consulting or Advisory Role: Roche, Bayer, Merck & Co. Inc., Astra Zeneca, Pfizer, Astellas/Seattle Genetics; Travel, Accommodations, Expenses:Roche, Merck & Co. Inc., Pierre Fabre, PeerVoice; Research Funding (Institution):Merck & Co. Inc., Astra Zeneca, Amgen
Joaquim Bellmunt: Consultant for Bayer, Sanofi, Pfizer, Novartis, Eisai, Janssen, Amgen, Astrazeneca, Merck, Genentech, Agensys, Seattle Genetics; Research support from Novartis an Sanofi; Author for UpTodate.
Jae-Lyun Lee: Consultant for Astellas, AstraZeneca, Eisai; Research funding from Pfizer, Janssen, Novartis, Exelixis; Honoraria from Pfizer, Astellas
Shih-Wen Lin, Joe Simpson, Christina Derleth: Employed by and own stocks in Genentech/Roche
Jonathan E. Rosenberg: consultant to Merck, Bristol-Myers Squibb, Eli Lilly, Agensys, Genentech (Roche), Sanofi, EMD Serono, AstraZeneca, Innovio, Seattle Genetics, Oncogenex, and Bayer; Owns stock in Merck and Illumina.
Toni K. Choueiri: research funding from Pfizer, Exelixis, BMS, Novartis, and has an advisory role at Pfizer, Novartis, Genentech, Merck, BMS and Bayer.
Dean Bajorin: consultancy for Bristol Myers Squibb, Genentech-Roche, Pfizer, Merck, Novartis and Eli-Lilly; research support from Bristol Myers Squibb, Genentech-Roche, Merck, Novartis and Amgen; and travel support from Eli-Lilly, Genentech and Merck.
Gregory Pond, Ashley Marie Regazzi, Stephanie A. Mullane, Daniele Raggi, Soonil Lee: None
References
- 1.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997;15:1853–1857. [DOI] [PubMed] [Google Scholar]
- 2.Roth BJ, Dreicer R, Einhorn LH, et al. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 1994;12:2264–2270. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–4461. [DOI] [PubMed] [Google Scholar]
- 4.Necchi A, Mariani L, Zaffaroni N, et al. Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol 2012;13:810–816. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 2006;24:3451–3457. [DOI] [PubMed] [Google Scholar]
- 6.Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int 2013;112:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol 2013;14:769–776. [DOI] [PubMed] [Google Scholar]
- 8.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–562. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017. [DOI] [PubMed]
- 10.Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 2016;34:3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017. [DOI] [PMC free article] [PubMed]
- 12.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonpavde G, Pond GR, Rosenberg JE, et al. Improved 5-Factor Prognostic Classification of Patients Receiving Salvage Systemic Therapy for Advanced Urothelial Carcinoma. J Urol 2016;195:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bambury RM, Benjamin DJ, Chaim JL, et al. The safety and efficacy of single-agent pemetrexed in platinum-resistant advanced urothelial carcinoma: a large single-institution experience. Oncologist 2015;20:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrylak DP PT, Bellmunt J, et al. Atezolizumab in patients with metastatic urothelial carcinoma (mUC): A 2-year clinical update from a phase Ia study. J Clin Oncol 35, no. 6_suppl (February 2017) 290–290. [Google Scholar]
- 16.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 2012;30:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II Study of Sunitinib in Patients With Metastatic Urothelial Cancer. Journal of Clinical Oncology 2010;28:1373–1379. [DOI] [PubMed] [Google Scholar]
- 18.Lee JL, Ahn JH, Park SH, et al. Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Invest New Drugs 2012;30:1984–1990. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Lee SI, Park SH, et al. A Phase II Study of Weekly Docetaxel as Second-Line Chemotherapy in Patients With Metastatic Urothelial Carcinoma. Clin Genitourin Cancer 2016;14:76–81. [DOI] [PubMed] [Google Scholar]
- 20.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013;105:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based Prediction of Overall Survival in Patients with Metastatic Urothelial Carcinoma Receiving First-line Platinum-based Chemotherapy: Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Eur Urol 2017;71:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, O’Donnell PH, Massard C, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol 2017:e172411. [DOI] [PMC free article] [PubMed]
- 23.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2017. [DOI] [PMC free article] [PubMed]
- 24.Powles T ea IMvigor211: A Phase III Randomized Study Examining Atezolizumab Versus Chemotherapy for Platinum-Treated Advanced Urothelial Carcinoma. EACR-AACR-SIC Special Conference June 24–27, 2017, Florence, Italy, abstract 606. [Google Scholar]
- 25.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010;28:1850–1855. [DOI] [PubMed] [Google Scholar]
- 26.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pond GR, Bellmunt J, Rosenberg JE, et al. Impact of the number of prior lines of therapy and prior perioperative chemotherapy in patients receiving salvage therapy for advanced urothelial carcinoma: implications for trial design. Clin Genitourin Cancer 2015;13:71–79. [DOI] [PubMed] [Google Scholar]
- 28.Pond GR, Agarwal N, Bellmunt J, et al. A nomogram including baseline prognostic factors to estimate the activity of second-line therapy for advanced urothelial carcinoma. BJU Int 2014;113:E137–143. [DOI] [PubMed] [Google Scholar]
- 29.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017. [DOI] [PubMed]