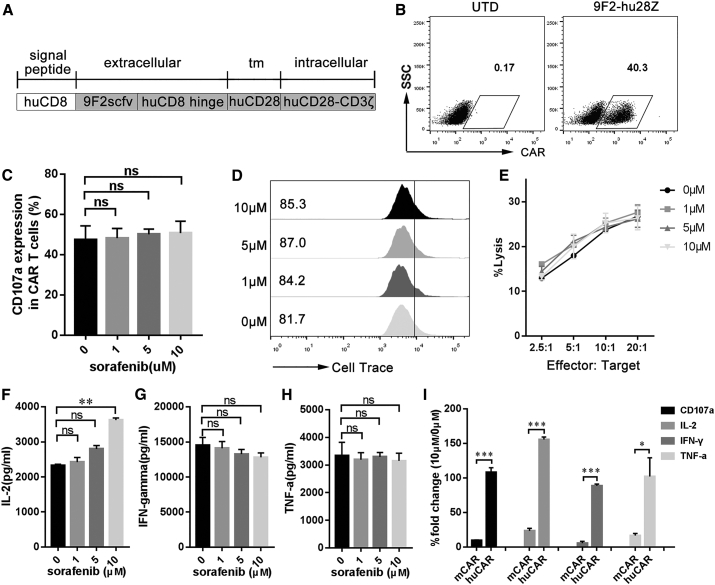
Figure 5.
Sorafenib Has No Significant Effect on the Activity of huCAR T Cells In Vitro
(A) Schematic representation of the modular composition of GPC3-specific human CAR. 9F2scFv, GPC3-specific single chain (heavy and light) fragment variable; tm, transmembrane domain; huCD28-CD3ζ, combined intracellular human CD28 and CD3ζ signaling domain. (B) Modified T cells express 9F2-hu28z CAR on their surface, based on flow cytometry results. (C) CD107a expression in activated huCAR T cells in the presence of sorafenib. huCAR T cells were stimulated with PLC/PRF/5 cells at 1:1 ratio in the presence of 1, 5, or 10 μM sorafenib for 24 h. Then, CD107a expression in huCAR T cells was assayed by flow cytometry. (D) The effect of sorafenib on huCAR T cell proliferation. huCAR T cells were prelabeled with Cell Trace Violet dye and then incubated with PLC/PRF/5 cells at a 1:1 ratio in the presence of different concentrations of sorafenib for 48 h. The numbers gated indicate the dividing cell population. (E) Cytotoxicity of sorafenib-pretreated huCAR T cells against tumor cells. huCAR T cells were pretreated with various concentrations of sorafenib for 4 h and then cocultured with PLC/PRF/5 cells at varying effector:target (E:T) ratios for 4 h for cytotoxic assays using a standard nonradioactive cytotoxic assay kit. The data are representative of three independent experiments. (F–H) In vitro cytokine production of huCAR T cells. Briefly, 1 × 105 huCAR T cells were cultivated in 24-well plates precoated with GPC3 protein. After 24 h, culture supernatants were harvested and assayed for IL-2 (F), IFN-γ (G), and TNF-α (H) via cytometric bead array (CBA). (I) Comparison of the activities (CD107a, IL-2, IFN-γ, and TNF-α) of mCAR and huCAR T cells after treatment with 10 μM sorafenib. All data are presented as the mean ± SEM of triplicate experiments, unless otherwise noted. Significance of findings was defined as follows: ns, not significant; p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.