Abstract
Mechanical thrombectomy has become the stand of care for patients with large vessel occlusions, yet major improvements in thrombectomy speed, efficacy, and completeness can still be achieved. High rates of clot fragmentation and failure to remove the clot resulting in poor neurological outcomes suggest that in order to further advance the field of stroke intervention; we must turn our attention towards understanding the science of clot. Accurately identifying the composition of the occlusive clot prior to intervention could significantly influence the success of the revascularization strategy used to treat them. Numerous features of thromboemboli could be studied and characterized, including quantitative histomorphometry and diagnostic imaging characteristics. Each of these features might logically predict superior thrombectomy outcomes with one device or another. This article aims to review the current literature on histopathological composition of acute ischemic stroke clots, with a particular focus on the correlation between clot composition and diagnostic imaging, stroke etiology and revascularization outcomes.
Keywords: Acute Ischemic Stroke, Blood Clot Composition, Histology Quantification, Mechanical Thrombectomy
Introduction
Mechanical thrombectomy has become the standard of care for many patients suffering from acute ischemic stroke following the publication of multiple randomized controlled trials that have demonstrated that endovascular revascularization, as compared to intravenous (IV) tissue plasminogen activator (t-PA) treatment alone, results in significantly higher rates of good neurological outcome and functional independence at 90 days [1–5]. More recently, the results of two further trials called DEFUSE 3 and DAWN were published, demonstrating the benefit of endovascular treatment in selected patients up to 16 and 24 hours, respectively, after time last seen well [2, 6]. When engaging a clot with a stent-retriever or suction catheter, optimal outcomes are likely best achieved when the entirety of the thrombus is able to be retrieved in one pass. Multiple passes indicate that the clot is adherent to the vessel or fragmented which could put the patient at risk for distal emboli and less than optimal recanalization outcomes [7]. It has been shown that the histological content of the clot influences the mechanical characteristics of thrombi and thus affect the ability of a stent-retriever device or suction catheter to engage it [8].
The advent of mechanical thrombectomy devices has created the unique opportunity to study acute ischemic stroke clot material. Accurate interpretation of the histologically stained slides has remained the foundation of pathological analysis and diagnostic medicine for over a century and studying the histomorphology of retrieved clots is crucial to improving our understanding of the variations in clot compositions of various etiologies. Cardioembolic clots often occur due to a new onset of a heart arrhythmia, typically atrial fibrillation and large artery clots tend to result from the embolization of atherosclerotic plaque from a large artery source, commonly the carotid arteries. The histopathologic signatures of the clots formed as a result of these very different underlying conditions should be recognizable and identifiable, both to histopathologists and also to sophisticated image analysis software programs. Numerous features of thromboemboli can be studied and characterized, including quantitative histomorphometry and diagnostic imaging characteristics [8–12] and each of these features might logically predict superior thrombectomy outcomes with one device or another. This article aims to review the current literature on histopathological clot composition, with a particular focus on the correlation between clot composition and imaging, stroke etiology and revascularization outcomes.
Histopathologic Analysis
Over the past several years, there has been an increase in the number of studies characterizing clots retrieved from patients with acute ischemic stroke [13–24]. However, in general, there is a lack of uniformity in the histopathologic characterization of retrieved thrombi [9]. H&E is the most widely used histology stain and is considered the Gold-standard for the diagnosis of many diseases including cancer and to date most studies analyzing clot composition have used the classical Hematoxylin and Eosin (H&E) stain to characterize AIS clot composition [12, 25, 26]. The major advantage of the H&E stain is that it is a relatively simple stain and results in dissimilar colours of cell nuclei and cytoplasm, allowing easy recognition of cells and cell populations. However, the major limitations of the H&E stain are that the cytoplasmic differentiation is insufficient, reticular fibres, basement membranes and cell borders are not stained and the contrast between cytoplasmic and other extracellular structures is poor. This means that studies that have used H&E for the characterization of AIS clots typically only identify three components of clots (Red Blood Cells, White Blood cells and Fibrin/Other) and have tended to classify the clots into sub-groups such as Red-Blood Cell-Rich, Fibrin-Rich and Mixed [24]. However, this fails to take into consideration other key factors in the coagulation cascade such as Platelets and von Willebrand Factor that have also been shown to be present at high levels in clots. Several more recent studies have acknowledged this and now refer to this sub-group as Fibrin/Platelet-Rich [27, 28]; however, the classification of clot composition using only H&E staining is still inadequate.
Masson’s trichrome is a three-colour staining protocol that has occasionally been used for the identification of AIS clots components [29, 30]. Masson’s trichrome is best suited to distinguishing cells from surrounding connective tissue and many of the components that it specifically identifies such as muscle fibres and bone are not commonly found in AIS clots. Hence it has more usefulness in the characterization of atherosclerotic plaque and could potentially be incorporated in the histological characterization of AIS clots when a Large Artery aetiology is suspected. Additionally, several studies have used other histological stains to study clot composition, such as Elastica von Gieson (Connective tissue), Prussian Blue (Iron) and Gomori trichrome stain (Muscle), but have not reported any significant correlations with imaging, etiology or outcome using these stains [13, 21, 31].
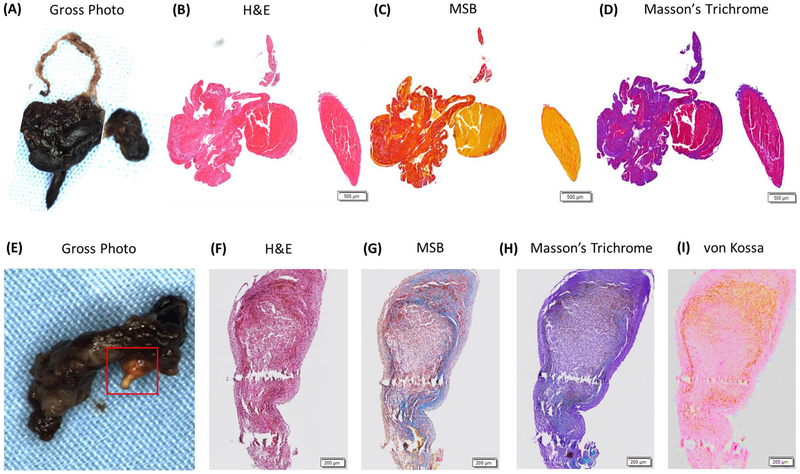
Martius Scarlett Blue (MSB) stain is another commonly used histological stain that has been used for the characterization of clot composition previously [10, 30, 32]. MSB staining allows for the identification of Red Blood cells (Yellow), Fibrin (Red), White Blood Cells (Purple/blue) and Collagen (Blue). The major advantage of the MSB stain is that the distinctive colour separation is much better than H&E and therefore enables more accurate quantification of clot components, as can be seen in Figure 1. The MSB stain also identifies more components that the Traditional H&E stain, with the identification of Collagen and consequently MSB seems to be the optimal histology stain for the identification of the major components of AIS Clots.
Figure 1: Commonly used histological stain in acute ischemic stroke clot assessment.
A&E are gross photographs of clots removed from two different patients that had suffered an acute ischemic stroke with an area of suspected calcification in E highlighted with a red box. B&F are examples of H&E stained slides from the corresponding clots showing Red Blood Cells (Red), White Blood Cells (Purple) and Fibrin/Platelets (Pink). C&G are examples of MSB stained slides showing Red Blood Cells (Yellow), White Blood Cells (Purple), Fibrin (Red) and Platelets/Other (Grey). D&H are examples of Masson’s Trichrome stained slide showing Red Blood Cells (Red), White Blood Cells (Purple) and Fibrin/Platelets (Purple). I is an example of a von Kossa stained slide confirming the presence of calcification (Brown).
The presence of calcification in AIS clots has been reported previously [30] and cases in which calcification is present are likely due to the embolization of atherosclerotic plaque from a large artery source [33, 34]. Calcified cerebral emboli are frequently overlooked or misinterpreted [35]. They are composed of large amounts of calcium phosphate which influences their mechanical properties and therefore, render them stiffer and less accessible for stent retrievers [33]. Areas of suspected calcification can often be identified using H&E, Masson’s Trichrome and MSB, however, the von Kossa stain is used to specifically identify mineralization in tissue and should be used to confirm the presence of calcification when suspected, as demonstrated in Figure 1. To date, no studies have shown a correlation between the presence of calcification as identified by von Kossa staining and large artery aetiology.
The advantages and disadvantages of histological stains commonly used to characterize the composition of stroke thrombi are summarized in Table 1.
Table 1:
Summary of the major advantages and disadvantages of histological staining techniques commonly used in the characterization of acute ischemic stroke clots.
| Histological Stains: | Advantages: | Disadvantages: |
|---|---|---|
| Hematoxylin and Eosin |
|
|
| Martius Scarlett Blue |
|
|
| Masson’s Trichrome |
|
|
| Von Kossa |
|
|
Immunohistochemical Analysis
In addition to the use of basic histological stains, many studies are now using immunohistochemistry to identify specific components of AIS clots. Platelets play a key role in response to endothelial injury as platelet plug formation is associated with activation of the coagulation cascade and resultant fibrin deposition. Platelet-rich clots have long been known to be more resistant to thrombolytic therapy [36, 37] and platelets have been shown to be present at similar levels to RBCs and Fibrin in AIS clots [14]. As shown in Table 2, various antibodies have been used to investigate platelet composition in clots. Von Willebrand factor (vWF), a large multimeric plasma glycoprotein, plays a major role in blood coagulation and it is important in platelet signaling promoting their adhesion to vascular injured sites. Importantly, vWF can be cleaved by the metalloprotease ADAMTS13 into smaller and less reactive multimers leading to thrombus dissolution. Neutrophil extracellular traps (NETs) promote thrombus formation generating a scaffold for platelets and RBCs thereby influencing the coagulation cascade [38]. NETs are fibrous networks of extracellular DNA released by neutrophils under the form of de-condensed chromatin associated with histones and neutrophil granule proteins such as myeloperoxidase and neutrophil elastase. Antibodies against myeloperoxidase (MPO) and citrullinated histones H3/H4 as well as neutrophils markers (CD66b and neutrophil elastase) have been used to assess thrombus composition [23, 38].
Table 2.
Summary of the main antibodies previously used for the assessment of acute ischemic stroke clot composition.
| Target: | Antibody Used: | References: |
|---|---|---|
| Platelets | GPIIb/IIIa (CD61), GPIb | [19, 41, 42] |
| CD31 (PECAM-1) | [43, 44] | |
| CD42b | [32] | |
| Von Willebrand Factor | vWF | [39, 45] |
| Fibrin | FibII | [19] |
| Endothelial Cells | CD34 | [30, 42] |
| Neutrophils | Neutrophil Elastase | [38, 40] |
| MPO | [23] | |
| CD66b | [38] | |
| NETs | H3Cit | [35] |
| Histon H4 | [23] | |
| T-Cells | CD3 | [22, 46] |
| B-Cells | CD20 | [22] |
| Macrophages | CD68 | [22, 39] |
Immune cells are known to be involved in thromboinflammation during stroke, including CD3+/CD4+ T cells and CD68+ monocytes [39] and it has been suggested that the degree of infiltration by inflammatory cells may impact the mechanical properties of the thrombus including its stability and degradation [40]. The interaction of immune cells with platelets (e.g., via cluster of differentiation 40 (CD40) and CD40 ligand or P-selectin (CD62-P) and P-selectin glycoprotein ligand 1 generating platelet-leukocytes complexes) and with endothelial cells (e.g., via intercellular adhesion molecule 1 and lymphocyte function-associated antigen 1) may play a role in thrombus formation [39]. Early endothelialization may occur over and within the thrombus affecting the clot dissolution by t-PA. Although endothelial cells covering the surface of thrombi is part of their normal evolution, t-PA will not penetrate the thrombus depending on the degree of endothelialization [30]. Therefore, endothelialization and calcification of clots are among the factors that may reduce t-PA efficacy.
Quantification Methods
Quantification methods of clot histologic characteristics are highly variable, with many studies relying on visual inspection rather than more robust techniques such as computer-aided quantification [9]. Whilst manual interpretation of histological and immunohistochemical stained slides remains the cornerstone of diagnosis in many diseases such as cancer, manual quantitative of composition has high inter-observer variability and low reproducibility [47]. The field of digital pathology has been around for some time and many image analysis software packages have been used with some success to quantify the components of clots from scanned H&E stained slides including ImageJ, Aperio and Adobe [24, 27, 28]. We are now entering the era of artificial intelligence and we believe that the use of Machine Learning will become commonplace in the analysis and quantification of histological and immunohistochemically stained images. Machine-learning based Image analysis software packages such as QuPath and Orbit Image Analysis have been used previously and allow for quick and accurate quantification of tissue components by using automated segmentation algorithms combined with trainable cell/tissue classification [48, 49]. The use of Machine-learning techniques will undoubtedly increase the accuracy and reproducibility of quantitative histopathology which will be crucial to determining the cellular composition of acute ischemic stroke clot components.
Clot Composition and Stroke Etiology
Initial studies examining correlations between stroke etiology and clot composition determined using H&E staining were conflicting, with studies showing no correlation, a correlation between RBC-Rich emboli and cardioembolic stroke and conversely a correlation between RBC-Rich emboli and large artery atherosclerosis [12–14, 16–19, 21, 25, 42]. These studies had relatively small cohorts of patients ranging from 17–54. A previous systematic review article on these studies by the authors found that there was no significant difference in the proportion of RBC-Rich thrombi between cardioembolic and large artery atherosclerosis etiologies [50]. However, a recently published study by Sporns et al that included 187 patients again suggested that Cardioembolic thrombi had significantly fewer erythrocytes and higher proportions of fibrin/platelets than non-cardioembolic thrombi [22], a finding supported by another more recent study [24]. Boeckh-Behrens et al performed a quantitative analysis of 145 H&E stained thrombi collected from stroke patients with large-vessel occlusion and found that cryptogenic strokes strongly overlap with cardioembolic strokes but not with non-cardioembolic strokes in terms of thrombus histology as well as interventional and clinical outcome parameters [51]. Interestingly a recent study has shown that a higher percentage of white blood cells (WBCs) in the thrombus was associated with cardioembolic etiology and hypothesize that WBC-mediated immunological and coagulatory processes may play a key role in thrombus formation and pathogenesis of stroke [12].
The amount of Platelets does not appear to be related to the aetiology as they appear to be present at similar levels in both cardioembolic and large-artery atherosclerotic patients [14, 19, 52]. However the distribution of platelets within the clot might be varied; Ahn et al observed that in arteriogenic clots platelets covered the fibrin layers or were localized at the edge or periphery of the clot whereas in cardiogenic thrombi, fibrin was most abundant and platelets were clustered within the fibrin-rich regions [32]. vWF-positive areas co-localize with regions of fibrin and collagen and inversely correlate with red blood cell content, thus vWF and platelets are suspected of being major components of ‘white’ clots [53, 54]. Schuhmann et al revealed that the number of CD4+ and CD68+ cells was increased in erythrocytic and red clots compared to white thrombi rich in vWF+ cells compared and mixed clots. However, the mechanisms by which the immune cells contribute to the pathogenesis of stroke are not completely understood [39]. It has also been demonstrated that neutrophils are abundantly present in thrombi of all pathogenesis [38], however, there are again conflicting studies on correlations between the number of NETs in AIS thrombi and stroke etiology. Laridan et al found a significant correlation between a higher amount of NETs in thrombi from cardiac origin compared with non-cardiac thrombi, whereas Ducroux et al showed that NETs are important constituents of thrombi irrespectively of their aetiology [23, 38].
The inconsistencies in the reported findings to date are likely due to differing interpretations and implementation of the TOAST criteria, diverse quantification methods and to the aforementioned limitations of the H&E stain. The authors suggest that studies including larger patient cohorts, the use of a more comprehensive histological stain such as the MSB stain and standardized quantification techniques are required before definitive correlations between histological composition and stroke aetiology can be confirmed.
Clot Composition and Outcome
As highlighted earlier, AIS clot histological compositions are diverse often as a result of their differing etiologies. It is therefore logical to assume that such diversity in composition will have a significant impact on revascularization outcome; yet, the neurovascular community still lacks a complete understanding of the distribution of clot phenotypes and their putative association with revascularization outcomes. Red Blood Cell-rich clots are associated with significantly higher recanalization rates, reduced number of maneuvers and a shorter mean recanalization time than fibrin-rich clots. This is likely due to the fact that Fibrin-rich clots have a significantly higher coefficient of friction than Red Blood Cell-rich clots and therefore have a stronger interaction with the vessel wall and are harder to remove from the vessel wall [55]. Additionally, the viscoelastic properties of clots change according to their composition, with Red Blood Cell-rich clots being more viscous, as water is the main constituent of RBCs, and fibrin-rich clots being more elastic [56]. Calcified thromboemboli are occasionally encountered (1% of patients) and calcified specimens are stiffer than arteriogenic and cardiogenic emboli, resulting in poorer revascularization outcomes [8, 33]. The amount of WBCs has also been shown to influence the success of the procedure [12]. A higher percentage of WBCs in the thrombus has been shown to be associated with an extended mechanical recanalization time, a less favorable recanalization rate (TICI score) and a poorer clinical outcome (NIHSS post treatment) suggesting that leukocytes are a notable factor in thrombus formation and development of acute cerebral ischemia [13].
The platelet cell adhesion molecule CD31 (PECAM-1) is a receptor protein expressed by multiple cell types involved in coagulation and immunological processes and is thought to have a neuroprotective effect. Boeckh-Behrens et al demonstrated a significant correlation between CD31+ cells and an improved outcome in 86 patients treated with mechanically thrombectomy [44]. The platelet-to-lymphocyte ratio (PLR) was introduced as a potential marker to determine increased inflammation and a high-PLR ratio is associated with a poorer recanalization rate and a larger infarcted area [57]. Similarly, high levels of vWF and low ADAMTS13 levels are correlated with an increased risk of stroke and worse outcome. Consequently it has been suggested that pre-treatment with anti-platelet therapy or targeting vWF directly by ADAMTS13 action or by inhibition of the vWF-platelet glycoprotein Ib (vWF-GPIb) interaction may become promising thrombolytic strategies and potentially improve the odds of a successful recanalization in patients treated with MT [58]. High amounts of neutrophil elastase-positive cells are also related to an increased risk of periprocedural thrombus fragmentation [40]. It is obvious that the composition of AIS clots affects the success of revascularization strategies and therefore in order to increase the rate of successful revascularization, we must first understand more about the histological composition of clots from different etiologies.
Imaging Analysis and Clot Characterization
Diagnostic Imaging, including non-contrast computed tomography (NCCT) computed tomography angiography (CTA) and magnetic resonance imaging (MRI), of AIS has evolved from a diagnostic tool to a quantitative and qualitative assessment tool that can help to predictive response to t-PA and mechanical thrombectomy. The presence of a Hyperdense Artery Sign (HAS) on non-contrast CT has been shown to indicate a Red Blood Cell-rich phenotype [25, 59] and is associated with improved recanalization rates [25, 50, 60–67]. Niesten et al performed CD31 immunohistochemistry and found a non-significant negative correlation between CT attenuation and the proportion of platelets [23].
A Susceptibility Vessel Sign (SVS) on MRI corresponds to a localized hypo intense signal at the site of the thrombus and is also related to the amount of red blood cells within the thrombus [25, 29]. There appears to be a consensus in the literature that the presences of an SVS sign is associated with an improved functional outcome but not an improved revascularization outcome [68–70]; Bourcier et al assessed patients from the Contact Aspiration vs Stent Retriever for Successful Revascularization (ASTER) and THRombectomie des Artères CErebrales (THRACE) trials and found that SVS was associated with lower disability at 3 months but mTICI scores did not differ between groups [68]. The proportion of platelets was significantly higher in clots with a negative susceptibility vessel sign compared to those with a positive susceptibility vessel sign. SVS identification is reliable and reproducible, however a major limitation is that the prevalence of the SVS+ varies significantly among MRI machines [71].
In addition to NCCT, most patients also undergo a CTA as part of their diagnostic imaging. The rate of uptake of the contrast agent by the clot, known as the permeability of the clot, can be quantified by the level of contrast penetration and has been shown to be related to the composition of the clot [72]. Thrombus Attenuation Increase from NCCT to CTA has previously been shown to be associated with improved functional outcomes in patients treated with rtPA and patients treated using mechanical thrombectomy [73, 74]. A recent study has suggested that permeable thrombi correlate with lower fractions of red blood cells counts and more fibrin/platelets conglomerations, concurrent with an association with cardioembolic origin [82]. The assessment of perviousness measures is easy to implement during admission imaging of patients with stroke that consists of non-contrast CT (NCCT) and CTA imaging and has the potential to give additional information about the occluding thrombus.
Future Directions
Proteomics promises to be a useful tool to identify blood-based biomarkers in acute ischemic stroke [75–77]. Targeted proteomics chips examining certain proteins have been found to predict incident ischemic stroke in two independent Swedish cohorts of adults aged over 70 years[78]. Some groups have used a brain proteomics approach to detect specific patterns of protein expression for different areas of brain tissue affected by stroke (core, penumbra and non-ischemic areas) [79].
The specific molecular composition of the retrieved clots is still largely unknown and therefore, clots retrieved by thrombectomy represent a resourceful material for proteomic characterization that may allow a better understanding of the molecular mechanisms of thrombus formation. Two recent studies have reported the use of mass spectrometry on frozen samples in the attempt not only to establish the peptide composition of emboli but also to discover potential biomarkers for stroke etiology [80, 81]. Rao et al performed mass spectrometry analysis of 20 thrombi retrieved from patients with different stroke etiologies in an attempt to identify specific peptides in clot composition corresponding to the tissue of origin. The study detected 81 common proteins in all 20 samples and that proteins associated with inflammation were found in emboli[80]. Munoz et al defined a reference proteome of 339 proteins detected in four studied thrombi. Further studies with larger cohorts of patients are needed and extensive validation must be carried out in order to implement these findings into the clinical practice. However, the preliminary results are encouraging and the availability of preserved clinical samples suggests that the use of mass spectrometry to identify potentially novel biomarkers of stroke subtype should be considered in the future. The ultimate utility of such analyses will be to provide insight into stroke etiology, particular in the group of patients with stroke of undetermined source.
Future Directions
Notwithstanding the many potential benefits of mechanical thrombectomy, major improvements in thrombectomy speed, efficacy, and completeness must be achieved before the full benefit of the procedure is realized by patients. Revascularization outcome is critical to achieving good neurological outcomes [82–84], however, data from the randomized controlled trials published in 2015 show that the rate of TICI 2b/3 recanalization is highly variable ranging, from 59%−88%, with most studies showing rates lower than 80% [85–89]. High rates of clot fragmentation and failure to remove the clot resulting in poor neurological outcomes suggest that accurately identifying the composition of the occlusive clot prior to intervention could significantly influence the success of the thrombectomy procedure. In order to further advance the field of stroke intervention, we must turn our attention towards the science of clot, by gaining a greater understanding of the distribution of clot phenotypes and their association with recanalization outcomes, we can arrive at a better understanding of why it is that successful recanalization is only achieved in 70–80% of cases and develop technologies and techniques that can be used to successfully retrieve these difficult clots.
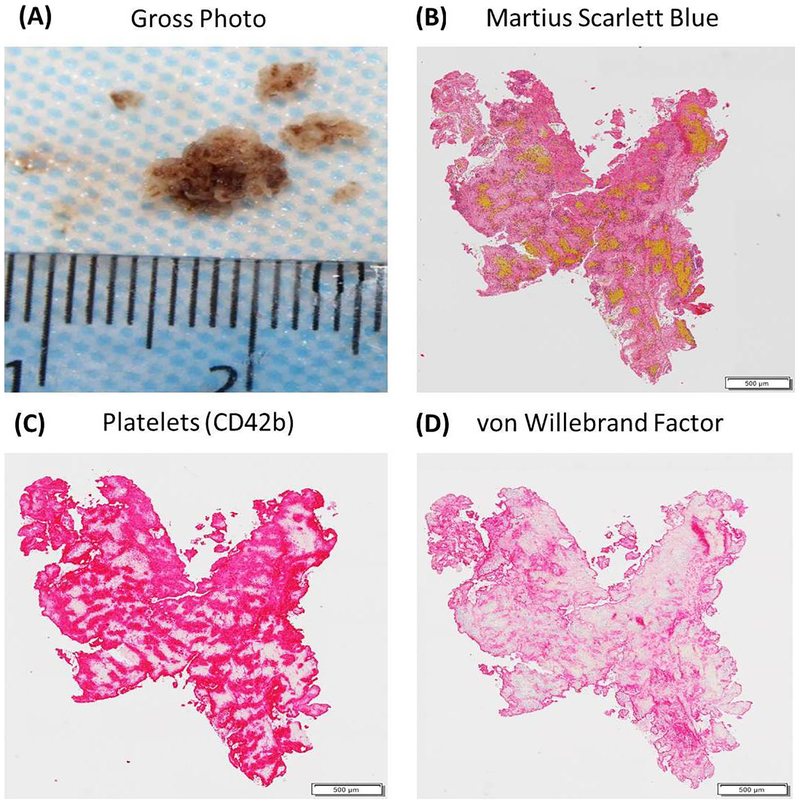
Figure 2: Histologic and Immunohistochemical Analysis of an acute ischemic stroke clot.
(A) is a gross photograph a ‘White’ clot retrieved from a patient using a mechanical thrombectomy procedure. (B) is an example of an MSB stained slide from the same clot showing the presence of Red Blood Cells (Yellow), White Blood Cells (Purple), Fibrin (Red) and Platelets/Other (Grey). (C) is an example of an immunohistochemically stained slide demonstrating the presence of Platelets in the clot (CD42b=Red). (D) is an example of an immunohistochemically stained slide demonstrating the presence of von Willebrand factor (Red).
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (R01 NS105853).
Disclosures: Waleed Brinjikji is CEO of Marblehead Medical LLC and has research funding and is a consultant for Johnson and Johnson. David Kallmes is President of Marblehead Medical LLC and has research funding from Styker and Johnson and Johnson
References
- 1.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T et al. : Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. New England Journal of Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA et al. : Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. New England Journal of Medicine 2017, 378(1):11–21. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M et al. : Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. New England Journal of Medicine 2015, 372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL et al. : Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. New England Journal of Medicine 2015, 372(11):1019–1030. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W et al. : Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. New England Journal of Medicine 2015, 372(24):2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T et al. : Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. New England Journal of Medicine 2018, 378(8):708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froehler MT, Tateshima S, Duckwiler G, Jahan R, Gonzalez N, Vinuela F, Liebeskind D, Saver JL, Villablanca JP, Investigators US: The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. Journal of Neurointerventional Surgery 2013, 5(4):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chueh JY, Wakhloo AK, Hendricks GH, Silva CF, Weaver JP, Gounis MJ: Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol 2011, 32(7):1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Meyer SF, Andersson T, Baxter B, Bendszus M, Brouwer P, Brinjikji W, Campbell BC, Costalat V, Davalos A, Demchuk A et al. : Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke 2017:1747493017709671. [DOI] [PubMed] [Google Scholar]
- 10.Duffy S, Farrell M, McArdle K, Thornton J, Vale D, Rainsford E, Morris L, Liebeskind DS, MacCarthy E, Gilvarry M: Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J Neurointerv Surg 2016. [DOI] [PubMed] [Google Scholar]
- 11.van der Marel K, Chueh JY, Brooks OW, King RM, Marosfoi MG, Langan ET, Carniato SL, Gounis MJ, Nogueira RG, Puri AS: Quantitative assessment of device-clot interaction for stent retriever thrombectomy. Journal of Neurointerventional Surgery 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeckh-Behrens T, Schubert M, Förschler A, Prothmann S, Kreiser K, Zimmer C, Riegger J, Bauer J, Neff F, Kehl V et al. : The Impact of Histological Clot Composition in Embolic Stroke. Clinical neuroradiology 2016, 26(2):189–197. [DOI] [PubMed] [Google Scholar]
- 13.Boeckh-Behrens T, Schubert M, Forschler A, Prothmann S, Kreiser K, Zimmer C, Riegger J, Bauer J, Neff F, Kehl V et al. : The Impact of Histological Clot Composition in Embolic Stroke. Clin Neuroradiol 2014. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS: Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am J Neuroradiol 2015, 36(9):1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK et al. : CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011, 42(5):1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R et al. : Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke; a journal of cerebral circulation 2006, 37(8):2086–2093. [DOI] [PubMed] [Google Scholar]
- 17.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, de Bruin PC, Mali WP, Velthuis BK: Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE 2014, 9(2):e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallustio F, Koch G, Di Legge S, Arno N, Sama D, Giordano A, Pampana E, Stefanini M, Konda D, Reale CA et al. : Histological features of thrombo-embolic specimens collected from intracranial arteries of patients with acute ischemic stroke. Cerebrovascular Diseases 2013, 35:180. [Google Scholar]
- 19.Sato Y, Ishibashi-Ueda H, Iwakiri T, Ikeda Y, Matsuyama T, Hatakeyama K, Asada Y: Thrombus components in cardioembolic and atherothrombotic strokes. Thrombosis research 2012, 130(2):278–280. [DOI] [PubMed] [Google Scholar]
- 20.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B: Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol 2015, 42(2):86–92. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner C: Cerebrovascular occlusive disease histopathological analysis of thrombi retrieved by endovascular mechanical extraction during acute ischemic stroke. International Journal of Stroke 2014, 9:143. [Google Scholar]
- 22.Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, Heindel W, Jeibmann A, Niederstadt TU: Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke 2017, 48(8):2206–2210. [DOI] [PubMed] [Google Scholar]
- 23.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Ben Maacha M, Blanc R, Redjem H, Ciccio G: Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018, 49(3):754–757. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, Yamasaki M, Kobayashi K, Sano T, Mori G: Erythrocyte-Rich Thrombus Is Associated with Reduced Number of Maneuvers and Procedure Time in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovascular diseases extra 2018, 8(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK et al. : CT and MRI Early Vessel Signs Reflect Clot Composition in Acute Stroke. Stroke 2011, 42(5):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi AI, Qureshi MH, Lobanova I, Bashir A, Khan AA, Bologna SM, Peterson M, Suri MFK: Histopathological Characteristics of IV Recombinant Tissue Plasminogen -Resistant Thrombi in Patients with Acute Ischemic Stroke. Journal of Vascular and Interventional Neurology 2016, 8(5):38–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Choi MH, Park GH, Lee JS, Lee SE, Lee S-J, Kim J-H, Hong JM: Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke 2018, 49(3):652–659. [DOI] [PubMed] [Google Scholar]
- 28.Maegerlein C, Friedrich B, Berndt M, Lucia KE, Schirmer L, Poppert H, Zimmer C, Pelisek J, Boeckh-Behrens T, Kaesmacher J: Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interventional Neuroradiology 2018, 24(1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, Nagatsuka K, Ishibashi-Ueda H, Kira J-i, Toyoda K: Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 2016, 47(12):3035–3037. [DOI] [PubMed] [Google Scholar]
- 30.Almekhlafi MA, Hu WY, Hill MD, Auer RN: Calcification and endothelialization of thrombi in acute stroke. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2008, 64(3):344–347. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Fernández F, Rojas-Bartolomé L, García-García J, Ayo-Martín Ó, Molina-Nuevo JD, Barbella-Aponte RA, Serrano-Heras G, Juliá-Molla E, Pedrosa-Jiménez MJ, Segura T: Histopathological and Bacteriological Analysis of Thrombus Material Extracted During Mechanical Thrombectomy in Acute Stroke Patients. Cardiovascular and interventional radiology 2017, 40(12):1851–1860. [DOI] [PubMed] [Google Scholar]
- 32.Ahn SH, Hong R, Choo IS, Heo JH, Nam HS, Kang HG, Kim HW, Kim JH: Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. International Journal of Stroke 2016, 11(9):1036–1044. [DOI] [PubMed] [Google Scholar]
- 33.Dobrocky T, Piechowiak E, Cianfoni A, Zibold F, Roccatagliata L, Mosimann P, Jung S, Fischer U, Mordasini P, Gralla J: Thrombectomy of calcified emboli in stroke. Does histology of thrombi influence the effectiveness of thrombectomy? Journal of neurointerventional surgery 2018, 10(4):345–350. [DOI] [PubMed] [Google Scholar]
- 34.Raghib MF, Mutzenbach JS, Rösler C, Otto F, Mc Coy M, Müller-Thies-Broussalis E, Pikija S: Acute treatment of stroke due to spontaneous calcified cerebral emboli causing large vessel occlusion. Journal of Clinical Neuroscience 2017. [DOI] [PubMed] [Google Scholar]
- 35.Walker B, Shah L, Osborn A: Calcified cerebral emboli, a “do not miss” imaging diagnosis: 22 new cases and review of the literature. American Journal of Neuroradiology 2014, 35(8):1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang I-K, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, May JW, Collen D: Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation 1989, 79(4):920–928. [DOI] [PubMed] [Google Scholar]
- 37.Fay W, Eitzman D, Shapiro A, Madison E, Ginsburg D: Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and-independent mechanisms. Blood 1994, 83(2):351–356. [PubMed] [Google Scholar]
- 38.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF: Neutrophil extracellular traps in ischemic stroke thrombi. Annals of neurology 2017, 82(2):223–232. [DOI] [PubMed] [Google Scholar]
- 39.Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P: Immunohistochemical analysis of cerebral thrombi retrieved by mechanical thrombectomy from patients with acute ischemic stroke. International journal of molecular sciences 2016, 17(3):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaesmacher J, Boeckh-Behrens T, Simon S, Maegerlein C, Kleine J, Zimmer C, Schirmer L, Poppert H, Huber T: Risk of thrombus fragmentation during endovascular stroke treatment. American Journal of Neuroradiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallustio F, Koch G, Di Legge S, Arno N, Sama D, Giordano A, Pampana E, Stefanini M, Konda D, Reale C: Histological features of thrombo-embolic specimens collected from intracranial arteries of patients with acute ischemic stroke In: CEREBROVASCULAR DISEASES: 2013. KARGER ALLSCHWILERSTRASSE 10, CH-4009 BASEL, SWITZERLAND: 180–180. [Google Scholar]
- 42.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B: Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. Journal of Neuroradiology 2015, 42(2):86–92. [DOI] [PubMed] [Google Scholar]
- 43.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, de Bruin PC, Mali WP, Velthuis BK: Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 2014, 9(2):e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boeckh-Behrens T, Kleine J, Kaesmacher J, Zimmer C, Schirmer L, Simon S, Poppert H: The CD31 molecule: a possible neuroprotective agent in acute ischemic stroke? Thrombosis journal 2017, 15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, François O, Andersson T, Deckmyn H, Scheiflinger F: ADAMTS13-mediated thrombolysis of t-PA resistant occlusions in ischemic stroke in mice. Blood 2016:blood-2015-2008-662650. [DOI] [PubMed] [Google Scholar]
- 46.Dargazanli C, Rigau V, Eker O, Bareiro CR, Machi P, Gascou G, Arquizan C, Ayrignac X, Mourand I, Corlobé A: High CD3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One 2016, 11(5):e0154945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, Puhan MA, Slankamenac K, Graf R, Clavien P-A: Assessment of Hepatic Steatosis by Expert Pathologists: The End of a Gold Standard. Annals of Surgery 2009, 250(5):691–697. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald S, Wang S, Dai D, Murphree D, Pandit A, Douglas A, Kadirvel R, Brinjikji W, Kallmes DF, Doyle KM: Machine-Learned Characterization of Acute Ischemic Stroke Clots Reveals a Correlation Between Clot Composition and HU Density on CT. 2018. [Google Scholar]
- 49.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG: QuPath: Open source software for digital pathology image analysis. Scientific reports 2017, 7(1):16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, Dippel DWJ, Siddiqui AH, Khatri P, Baxter B et al. : Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. Journal of NeuroInterventional Surgery 2017, 9(6):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, Schirmer L, Nguyen K, Karatas D, Poppert H: Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 2016:STROKEAHA. 116.013105. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Yoon W, Kim T, Kim H, Heo T, Park M: Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. American Journal of Neuroradiology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuhmann KM, Gunreben I, Kleinschnitz C, Kraft P: Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke. International Journal of Molecular Sciences 2016, 17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prochazka V, Jonszta T, Czerny D, Krajca J, Roubec M, Macak J, Kovar P, Kovarova P, Pulcer M, Zoubkova R: The Role of von Willebrand Factor, ADAMTS13, and Cerebral Artery Thrombus Composition in Patient Outcome Following Mechanical Thrombectomy for Acute Ischemic Stroke. Medical Science Monitor 2018, 24:3929–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA: Clot friction variation with fibrin content; implications for resistance to thrombectomy. Journal of NeuroInterventional Surgery 2018, 10(1):34. [DOI] [PubMed] [Google Scholar]
- 56.Gersh KC, Nagaswami C, Weisel JW: Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and haemostasis 2009, 102(6):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altintas O, Altintas MO, Tasal A, Kucukdagli OT, Asil T: The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurological Research 2016, 38(9):759–765. [DOI] [PubMed] [Google Scholar]
- 58.Pandhi A, Tsivgoulis G, Krishnan R, Ishfaq MF, Singh S, Hoit D, Arthur AS, Nickele C, Alexandrov A, Elijovich L et al. : Antiplatelet pretreatment and outcomes following mechanical thrombectomy for emergent large vessel occlusion strokes. Journal of NeuroInterventional Surgery 2017. [DOI] [PubMed] [Google Scholar]
- 59.Ahn S, Choo I, Hong R: Hyperdense arterial sign reflects the proportion of red blood cells in the thromboemboli of acute stroke patients. Cerebrovasc Dis 2012, 33:236. [Google Scholar]
- 60.Kim EY, Heo JH, Lee SK, Kim DJ, Suh SH, Kim J, Kim DI: Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology 2006, 67(10):1846. [DOI] [PubMed] [Google Scholar]
- 61.Froehler MT, Tateshima S, Duckwiler G, Jahan R, Gonzalez N, Vinuela F, Liebeskind D, Saver JL, Villablanca JP: The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointerv Surg 2013, 5(4):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, Dowd CF, Higashida RT, Halbach VV, Hetts SW: Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44(1):243–245. [DOI] [PubMed] [Google Scholar]
- 63.Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI: Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. Journal of NeuroInterventional Surgery 2015, 7(2):104. [DOI] [PubMed] [Google Scholar]
- 64.Niesten JM, van der Schaaf IC, van der Graaf Y, Kappelle LJ, Biessels GJ, Horsch AD, Dankbaar JW, Luitse MJA, van Seeters T, Smit EJ et al. : Predictive Value of Thrombus Attenuation on Thin-Slice Non-Contrast CT for Persistent Occlusion after Intravenous Thrombolysis. Cerebrovascular Diseases 2014, 37(2):116–122. [DOI] [PubMed] [Google Scholar]
- 65.Puig J, Pedraza S, Demchuk A, Daunis-i-Estadella J, Termes H, Blasco G, Soria G, Boada I, Remollo S, Baños J et al. : Quantification of Thrombus Hounsfield Units on Noncontrast CT Predicts Stroke Subtype and Early Recanalization after Intravenous Recombinant Tissue Plasminogen Activator. American Journal of Neuroradiology 2012, 33(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jagani M, Kallmes DF, Brinjikji W: Correlation between clot density and recanalization success or stroke etiology in acute ischemic stroke patients. Interventional Neuroradiology 2017, 23(3):274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SK, Baek BH, Lee YY, Yoon W: Clinical implications of CT hyperdense artery sign in patients with acute middle cerebral artery occlusion in the era of modern mechanical thrombectomy. Journal of Neurology 2017, 264(12):2450–2456. [DOI] [PubMed] [Google Scholar]
- 68.Bourcier R, Hassen WB, Soize S, Roux P, Labreuche J, Kyheng M, Tisserand M, Rosso C, Blanc R, Piotin M et al. : Susceptibility vessel sign on MRI predicts better clinical outcome in patients with anterior circulation acute stroke treated with stent retriever as first-line strategy. Journal of NeuroInterventional Surgery 2018. [DOI] [PubMed] [Google Scholar]
- 69.Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, Serre I, Ben Hassen W, Zuber M, Calvet D et al. : Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. European Journal of Neurology 2015, 22(6):967–972. [DOI] [PubMed] [Google Scholar]
- 70.Bourcier R, Derraz I, Delasalle B, Beaumont M, Soize S, Legrand L, Desal H, Bracard S, Naggara O, Oppenheim C et al. : Susceptibility Vessel Sign and Cardioembolic Etiology in the THRACE Trial. Clin Neuroradiol 2018. [DOI] [PubMed] [Google Scholar]
- 71.Bourcier R, Détraz L, Serfaty JM, Delasalle BG, Mirza M, Derraz I, Toulgoat F, Naggara O, Toquet C, Desal H: MRI Interscanner Agreement of the Association between the Susceptibility Vessel Sign and Histologic Composition of Thrombi. Journal of Neuroimaging 2017, 27(6):577–582. [DOI] [PubMed] [Google Scholar]
- 72.Borggrefe J, Kottlors J, Mirza M, Neuhaus VF, Abdullayev N, Maus V, Kabbasch C, Maintz D, Mpotsaris A: Differentiation of Clot Composition Using Conventional and Dual-Energy Computed Tomography. Clinical neuroradiology 2017. [DOI] [PubMed] [Google Scholar]
- 73.Santos Emilie MM, Marquering Henk A, den Blanken Mark D, Berkhemer Olvert A, Boers Anna MM, Yoo Albert J, Beenen Ludo F, Treurniet Kilian M, Wismans C, van Noort K et al. : Thrombus Permeability Is Associated With Improved Functional Outcome and Recanalization in Patients With Ischemic Stroke. Stroke 2016, 47(3):732–741. [DOI] [PubMed] [Google Scholar]
- 74.Santos Emilie MM, Dankbaar Jan W, Treurniet Kilian M, Horsch Alexander D, Roos Yvo B, Kappelle LJ, Niessen Wiro J, Majoie Charles B, Velthuis B, Marquering Henk A et al. : Permeable Thrombi Are Associated With Higher Intravenous Recombinant Tissue-Type Plasminogen Activator Treatment Success in Patients With Acute Ischemic Stroke. Stroke 2016, 47(8):2058–2065. [DOI] [PubMed] [Google Scholar]
- 75.Piccardi B, Giralt D, Bustamante A, Llombart V, Garcia-Berrocoso T, Inzitari D, Montaner J: Blood markers of inflammation and endothelial dysfunction in cardioembolic Stroke: Systematic review and meta-analysis. Biomarkers 2017, 22(3–4):200–209. [DOI] [PubMed] [Google Scholar]
- 76.Prentice RL, Paczesny S, Aragaki A, Amon LM, Chen L, Pitteri SJ, McIntosh M, Wang P, Busald TB, Hsia J: Novel proteins associated with risk for coronary heart disease or stroke among postmenopausal women identified by in-depth plasma proteome profiling. Genome Medicine 2010, 2(7):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maestrini I, Ducroquet A, Moulin S, Leys D, Cordonnier C, Bordet R: Blood biomarkers in the early stage of cerebral ischemia. Revue neurologique 2016, 172(3):198–219. [DOI] [PubMed] [Google Scholar]
- 78.Lind L, Siegbahn A, Lindahl B, Stenemo M, Sundström J, Ärnlöv J: Discovery of new risk markers for ischemic stroke using a novel targeted proteomics chip. Stroke 2015:STROKEAHA. 115.010829. [DOI] [PubMed] [Google Scholar]
- 79.Cuadrado E, Rosell A, Colomé N, Hernández-Guillamon M, García-Berrocoso T, Ribo M, Alcazar A, Ortega-Aznar A, Salinas M, Canals F: The proteome of human brain after ischemic stroke. Journal of Neuropathology & Experimental Neurology 2010, 69(11):1105–1115. [DOI] [PubMed] [Google Scholar]
- 80.Rao NM, Capri J, Cohn W, Abdaljaleel M, Restrepo L, Gornbein JA, Yong WH, Liebeskind DS, Whitelegge JP: Peptide composition of stroke causing emboli correlate with serum Markers of atherosclerosis and inflammation. Frontiers in neurology 2017, 8:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muñoz R, Santamaría E, Rubio I, Ausín K, Ostolaza A, Labarga A, Roldán M, Zandio B, Mayor S, Bermejo R: Mass Spectrometry-Based Proteomic Profiling of Thrombotic Material Obtained by Endovascular Thrombectomy in Patients with Ischemic Stroke. International journal of molecular sciences 2018, 19(2):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rizvi A, Seyedsaadat SM, Murad MH, Brinjikji W, Fitzgerald ST, Kadirvel R, Rabinstein AA, Kallmes DF: Redefining ‘success’: a systematic review and meta-analysis comparing outcomes between incomplete and complete revascularization. Journal of NeuroInterventional Surgery 2018. [DOI] [PubMed] [Google Scholar]
- 83.Berkhemer OA, Fransen PS, Beumer D, Van Den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ: A randomized trial of intraarterial treatment for acute ischemic stroke. New England Journal of Medicine 2015, 372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 84.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W: Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New England Journal of Medicine 2015, 372(24):2285–2295. [DOI] [PubMed] [Google Scholar]
- 85.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ et al. : A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine 2015, 372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 86.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ et al. : Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. The New England journal of medicine 2015. [DOI] [PubMed] [Google Scholar]
- 87.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL et al. : Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. The New England journal of medicine 2015. [DOI] [PubMed] [Google Scholar]
- 88.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M et al. : Thrombectomy within 8 hours after symptom onset in ischemic stroke. The New England journal of medicine 2015, 372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 89.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W et al. : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. The New England journal of medicine 2015, 372(24):2285–2295. [DOI] [PubMed] [Google Scholar]