Abstract
Background:
Falls and fall risk factors are common among people living with HIV (PLWH). We sought to identify fall risk factors among men with and without HIV.
Methods:
Men aged 50–75 with (n=279) and without HIV (n=379) from the Bone Strength Substudy of the MACS were included. Multinomial logistic regression models identified risk factors associated with falling.
Results:
114 (41%) PLWH and 149 (39%) of uninfected men had ≥1 fall; 54 (20%) PLWH and 66 (17%) of uninfected men experienced ≥2 falls over 2 years. Five and 3% of PLWH and uninfected men, respectively, had a fall-related fracture (p=0.34). In multivariate models, the odds of ≥2 falls were greater among men reporting illicit drug use, taking diabetes or depression medications, and with peripheral neuropathy; obesity was associated with a lower risk (all p<0.05). In models restricted to PLWH, detectable plasma HIV-1 RNA, current use of efavirenz or diabetes medications, illicit drug use, and peripheral neuropathy were associated with a greater odds of having ≥2 falls (p<0.05). Current efavirenz use was associated with increased odds of an injurious fall; longer duration of antiretroviral therapy was protective (both p<0.05). Greater physical activity was associated with lower risk of falls with fracture (p<0.05).
Conclusions:
Identified risk factors for recurrent falls or fall with fracture included low physical activity, detectable HIV-1 RNA, use of efavirenz, or use of medications to treat diabetes and depression. Fall risk reduction should prioritize interventions targeting modifiable risk factors including increased physical activity, ART adherence, and transition off efavirenz.
Background
Among older adults, falls are the leading cause of injury-related morbidity and mortality1. Falls contribute to hospitalizations, and lead to limitations in activity as a consequence of fear of future falls2,3. Multiple factors can contribute to an increased risk for falls including multimorbidity, polypharmacy, neuropathy, cognitive impairment, impaired balance, and frailty4,5. Since aging people living with HIV (PLWH) have a higher burden of many of these risk factors for falls6,7, it is not surprising that the rate of falls among middle-aged PLWH (45–65 years old) is similar to the rate (25–30% per year) reported for all U.S. adults aged 65 and older4. Moreover, these rates appear to increase with age among older PLWH, with over 46% of 60–80 year old PLWH having experienced at least one fall in the prior year8. The frequency of falls in cohorts of demographically similar adults both with and without HIV (specifically the Multicenter AIDS Cohort Study [MACS] and the Women’s Interagency HIV Study [WIHS]) is similar to or higher than that of older adults in the general population9,10 Within the MACS and WIHS, falls have not been significantly higher among PLWH9,10. Participants in these prior analyses, however, were primarily middle-aged, with a mean age less than 509,11. Whether fall risks differ by HIV serostatus or effective HIV suppression among older adults is not known.
In prior studies of PLWH, falls were collected retrospectively which may underestimate the actual number of falls, may be more biased towards more severe falls, and may be less likely to capture details regarding the circumstances surrounding the fall compared to real-time reporting12,13. Identification of the circumstances surrounding a fall can distinguish between falls with high risk for a poor outcome (such as an indoor fall), or falls that are more likely the result of being active (i.e. while hiking or jogging) and less predictive of future functional status14. Collecting real-time fall characteristics can identify high p riority areas for interventions in falls-risk reduction.
Although any fall should trigger a clinical evaluation for modifiable risks, a major goal in fall reduction is reducing falls that result in a fracture. Impaired physical function and low bone density (a marker of fracture risk) are both associated with self-reported falls among PLWH4,15. Thus older adults with physical function impairments are likely at the greatest risk for both sustaining a fall and experiencing a fracture following a fall16. Whether these associations differ by HIV serostatus, particularly with the potential effects of antiretroviral therapy (ART), immunosuppression, and age on bone and muscle, are unknown.
The goals of this analysis were to identify common and unique fall risk factors (including physical function and balance measures) among older PLWH and men without HIV infection through prospective collection of falls, and to further explore the risk factors associated with fall complications.
Methods
Study Population
Men with and without HIV who participated in the Bone Strength Substudy (BOSS) of the MACS were included in this analysis. The MACS is an ongoing study of men who have sex with men, with or at risk for HIV infection at four sites in the United States: Los Angeles, CA; Pittsburgh, PA; Baltimore, MD/Washington D.C; and Chicago, IL. MACS participants return semi-annually for a standardized interview, clinical evaluation and laboratory tests. The BOSS Substudy included 658 MACS participants aged 50–79 years with a plasma HIV-1 RNA <200 copies/mL within 6 months of enrollment, and was initiated to investigate the contributions of aging, chronic HIV infection, and ART use on both skeletal and non-skeletal risk factors for fracture. The substudy was conducted between September 2012 and April 2015, with “baseline” in this analysis referring to the baseline BOSS assessment. Additional details have been previously published17. The institutional review boards at each site approved the study, and each participant provided written informed consent.
Outcome: Falls
A fall was defined as an event, including a slip or trip, in which a participant lost their balance and landed on the floor or ground or lower level, or hit an object like a table or chair. Participants were asked to report each fall in real-time (within 24 hours) for two years. After a fall, participants were instructed to call their MACS site (phone number provided on a wallet card) where site staff used a standardized fall reporting tool to gather information about the fall, including a description of the fall and whether it was due to external force, a seizure or loss of consciousness, its relation to consumption of alcohol, sleep medications or illicit substance use, whether the fall was attributed to low light, slippery conditions, uneven surface or obstacle, fatigue or weakness. Participants also reported whether they received medical care following the fall (fall with injury), and whether the fall resulted in a fracture. Participants were reminded by email, text, or phone every two months to report falls and were asked about any falls they may not have reported at semi-annual study visits. Two reviewers (KME and TTB) independently reviewed all reported falls and determined fall relationship to a pet, snow/ice, or a curb/step. Duplicate falls, falls that occurred when a participant was not on his feet (i.e., off a bicycle), and falls due to loss of consciousness or overwhelming force were excluded. For this analysis, we characterized types of falls and people who did or did not fall, with participants categorized as having 0, 1 (single faller), or ≥2 falls (recurrent faller) during the two-years.
Baseline risk factors
Physical function outcomes included a modified version of the Short Physical Performance Battery (mSPPB)18, functional reach, frailty, and the Activities-Specific Balance Confidence (ABC) survey. The mSPPB battery has been shown to improve discrimination of physical function at the higher end of the functional spectrum by using 10 repeat chair stands (increased from 5), a 4-m timed walk at usual speed, and a standing balance test for 30 seconds (increased from 10), with the addition of a single leg stand. The functional reach measured the distance a participant could extend his arm forward from a standing position without shifting his weight on his feet19. Frailty was assessed using criteria established by Fried, et al20, as previously used in the MACS Cohort7. Participants were considered non-frail if they met no components, pre-frail if 1 or 2 components were met, and frail if ≥3 components were met. The ABC survey assessed confidence in completing 16 tasks without losing balance or becoming unsteady. An average of balance confidence on each measure was calculated, with 100% indicating complete confidence21. Six items were also examined separately in a short-form (ABC-6)22. Two-cut points were examined, i.e. >80% and >90% confidence23,24.
Body mass index (BMI) categories included underweight/normal (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), obese (≥30 kg/m2). Depressive symptoms were defined by a score of ≥16 on the Center for Epidemiological Studies-Depression (CES-D) questionnaire; hepatitis B virus (HBV) infection by positive HBV surface antigen, and hepatitis C virus (HCV) by presence of serum viral RNA; liver disease by serum aspartate or alanine transaminase >150 u/L; diabetes mellitus as a fasting glucose ≥126 mg/dL or the self-reported diagnosis of diabetes with use of medications for glucose control; kidney disease by estimated glomerular filtration rate (GFR) <60mL/min/1.73 m2 body surface area using the Modification of Diet in Renal Disease25 equation; and hypertension as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or self-reported diagnosis of hypertension with use of anti-hypertensive medications. Polypharmacy was defined as 5 or more non -ART medications; use of controlled pain medications (i.e., opioids) or benzodiazepines were also considered. Neuroimpairment was defined as performing two standard deviations worse than HIV-uninfected age-, sex- and education-matched persons on two of three screening tests including Trails A, Trails B, and the Symbol Digit Modality Test26. Peripheral neuropathy was defined as <10 seconds perception of vibration at either great toe using a 120-Hz tuning fork27. HIV-specific factors included current CD4+ T-lymphocyte count/mm3 (CD4), plasma HIV-1 RNA level with suppression defined as <50 copies/mL, CD4 nadir, cumulative years of ART use, and any years of use of the following specific antiretroviral classes or agents: stavudine (D4T); didanosine (DDI); protease inhibitors (PI); tenofovir disoproxil fumarate (TDF); and efavirenz (EFV). We also calculated HIV RNA viral copy-years28, as a construct of viral load burden over the two-year fall data collection period, and dichotomized this distribution at the 75th percentile to classify men with high or low viral-copy years. Physical activity level was self-reported as low, moderate, or high, and measured using the International Physical Activity Questionnaire (IPAQ)29.
Statistical Analysis
Continuous and categorical data were reported as median with interquartile ranges (IQR) and frequency with column percentage, respectively. The distributions of demographics, behaviors, and morbidities by HIV serostatus were compared using Wilcoxon rank sum or Chi-square tests. Characteristics associated with the faller status (0, 1, 2+) was considered the primary outcome; characteristics associated with a fall, and characteristics associated with faller status with injury, or faller with fracture were secondary outcomes. Fall characteristics are described overall, and compared by HIV serostatus using Chi-square tests. Analyses exploring falls by curb/stairs or pets were post-hoc, and therefore information on pet ownership was not available for all men.
We used multinomial logistic regression to model faller status; demographics (age, race, site, and enrollment period), HIV serostatus, and BMI were included in the model. IIlicit drug use, peripheral neuropathy, and use of medications to treat depression, diabetes, and hypertension were risk factors selected for inclusion the final model by employing a stepwise, backward selection procedure (with p-value ≤0.2) for each the primary and secondary outcomes.
The faller with injury or fracture was modelled in a similar way using logistic regression, with the exception of no separate models by HIV serostatus for fallers with fracture due to small numbers. HIV factors were explored in models with PLWH only.
Associations of physical function measures (mSPPB and its components, functional reach, frailty, and ABC) with faller status were analyzed in separate models using multinomial logistic regression. A p-value of <0.05 was used to guide statistical interpretation. All analyses were completed using SAS v9.4 (SAS Institute, Cary, NC).
Results
Prospective falls over a two-year period were collected on 279 PLWH and 379 men without HIV. Baseline characteristics of these men are shown in Table 1. PLWH tended to be younger, black, to have a lower BMI and greater prevalence of diabetes and depression medication use, and differed by study site. Of 709 reported falls, 526 falls among 263 men met the study definition (exclusions were due to duplicate reporting, loss of consciousness, overwhelming external force, not being on feet at time of fall, or occurring outside of the reporting window).
Table 1.
Baseline Characteristics of the Study Population
| Men with HIV (n=279) | Men without HIV (n=379) | P-value | |
|---|---|---|---|
| Age | 61.1 (55.6,64.2) | 62.4 (58.5,66.8) | <0.001 |
| Race | |||
| White | 199(71%) | 312(82%) | 0.001 |
| Black | 67(24%) | 47(12%) | |
| Other | 13(5%) | 20(5%) | |
| Study Site | |||
| Baltimore | 64(23%) | 93(25%) | 0.005 |
| Chicago | 87(31%) | 73(19%) | |
| Pittsburgh | 64(23%) | 109(29%) | |
| Los Angeles | 64(23%) | 104(27%) | |
| Late cohort (enrolled after 2001) | 72(26%) | 50(13%) | <0.001 |
| Smoking status | |||
| Non-smoker | 78 (32%) | 131 (35%) | 0.57 |
| Former smoker | 143 (52%) | 195 (52%) | |
| Current smoker | 45 (16%) | 51 (14%) | |
| Physical activity | 0.77 | ||
| Low | 58(21%) | 80(21%) | |
| Moderate | 75(27%) | 109(29%) | |
| High | 145(52%) | 185(49%) | |
| Body mass index | 25.2(22.8,28.4) | 26.1(23.5,29.9) | 0.004 |
| Diabetes | 45(18%) | 42(12%) | 0.034 |
| Controlled (HbA1C <7.5%) | 37 (13%) | 32 (9%) | |
| Uncontrolled (HbA1C ≥ 7.5% | 7 (3%) | 12 (3%) | |
| Diabetes medications | 37(13%) | 37(10%) | 0.16 |
| Non-insulin therapy | 28 (10%) | 28 (7%) | |
| Insulin | 10 (4%) | 10 (3%) | |
| Depression medications | 81(30%) | 67(18%) | <0.001 |
| Illicit drug use | 130(48%) | 157(42%) | 0.14 |
| Peripheral neuropathy | 135(51%) | 143(40%) | 0.48 |
| Neuroimpairment | 15(7%) | 15(5%) | 0.51 |
| HIV-1 RNA <50 copies/mL | 255(91%) | - | |
| CD4 ≥ 500 cells/uL | 201(73%) | - | |
| Nadir CD4 <200 cells/uL | 101(36%) | - | |
| Current use of efavirenz | 30(11%) | - | |
| ABC ≤ 80 | 25 (9%) | 28 (7%) | 0.48 |
| ABC ≤ 90 | 66 (24%) | 81 (21%) | 0.51 |
| ABC-6 ≤ 80 | 55 (20%) | 71 (19%) | 0.78 |
| ABC-6 ≤ 90 | 116 (42%) | 153 (41%) | 0.80 |
| Tandem Stand (unable) | 126 (45%) | 149 (29%) | 0.13 |
| Functional Reach | 30.7 (25.5, 36.2) | 31.8 (26.1, 37.0) | 0.32 |
| 10-time chair rise | 23.9 (19.1, 26.8) | 23.1 (19.2, 27.6) | 0.84 |
| Grip Strength | 37 (30, 41) | 35 (30, 41) | 0.24 |
| Gait speed | 3.7 (3.4, 4.2) | 3.8 (3.3, 4.1) | 0.15 |
| SPPB Score <10 | 41 (15%) | 51 (13%) | 0.65 |
| Frailty Score | 0.09 | ||
| Non-frail | 158 (57%) | 235 (62%) | |
| Pre-frailty | 102 (37%) | 131 (35%) | |
| Frailty | 19 (7%) | 13 (3%) |
ABC, activities-specific balance confidence; SPPB, short physical performance battery
Fall Characteristics
Of PLWH, 114 (41%) reported at least one fall compared to 149 (39%) of uninfected men during the two-year study. Twenty-two (10%) falls among PLWH and 32 (11%) falls among men without HIV (p=0.56) were complicated by an injury leading to medical evaluation. Falls were complicated by fracture in 5% PLWH and 3% of men without HIV (p=0.34). Falls occurred more commonly in the winter, and were commonly attributed to an uneven or slippery surface and stairs or a curb. Falls were more likely to involve illicit substance use among men without HIV, and more likely related to a pet among PLWH. Other fall circumstances did not differ by HIV serostatus (Table 2).
Table 2.
Characteristics of the Falls Among Fallers with and without HIV
| Total number of Falls (n=526) | Falls among Men with HIV (n=234) | Falls among Men without HIV (n=292) | P-value | |
|---|---|---|---|---|
| Fall with injury | 54 (10.3%) | 22(9.4%) | 32(11%) | 0.56 |
| Fall with fracture | 20 (3.8%) | 11(4.7%) | 9(3.1%) | 0.34 |
| Winter | 160 (30.4%) | 71(30.3%) | 89(30.5%) | 0.97 |
| Fall during usual activities | 151 (28.7%) | 74(31.6%) | 77(26.4%) | 0.19 |
| Mentioned pet | 33 (6.3%) | 22(9.4%) | 11(3.8%) | 0.008 |
| Mentioned stairs/curb | 123 (23.4%) | 56(23.9%) | 67(22.9%) | 0.79 |
| Mentioned snow or ice | 77 (14.6%) | 40(17.1%) | 37(12.7%) | 0.15 |
| Attributed to lack of lighting | 60 (11.7%) | 29(12.8%) | 31(10.9%) | 0.51 |
| Attributed to a slippery surface | 141 (27.5%) | 64(28.2%) | 77(27%) | 0.77 |
| Attribute to an uneven surface | 174 (33.7%) | 79(34.5%) | 95(33.1%) | 0.74 |
| Attribute to an obstacle | 93 (18.1%) | 45(19.7%) | 48(16.8%) | 0.39 |
| Attributed to fatigue | 50 (9.8%) | 26(11.5%) | 24(8.4%) | 0.25 |
| Attributed to alcohol | 20 (3.9%) | 8(3.5%) | 12(4.2%) | 0.69 |
| Attributed to sleep medications | 97 (18.4%) | 47 (20.9%) | 50 (17.4%) | 0.31 |
| Attributed to illicit substance | 10 (2%) | 1(0.4%) | 9(3.2%) | 0.03 |
| Attributed to weakness | 91 (17.8%) | 44(19.4%) | 47(16.5%) | 0.40 |
Men who had fallen were stratified by HIV serostatus and, among PLWH, by baseline virologic suppression. Among the 23 men with detectable baseline HIV-1 RNA, the majority (65%) had an HIV-1 RNA between 50–200 copies/mL, 3 (13%) had a viral load 200–500 copies/mL, and 5 (22%) had an HIV-1 RNA of >500 copies/mL (4 with VL >10,000 copies/mL). Ninety-three men (33%) had a detectable HIV-1 RNA at any point during the 2 years of follow-up. PLWH with suppressed HIV-1 RNA and men without HIV experienced similar rates of falls. Although PLWH with a detectable HIV-1 RNA tended to have more falls and falls with injury or fracture, these differences did not reach statistical significance (all p>0.13).
Characteristics Associated with Single or Recurrent Fallers
The unadjusted odds of having fallen among all men or PLWH only are shown in Tables 3a and 3b. The odds of sustaining a single fall differed across study sites and enrollment cohorts, and were associated with diabetes, use of diabetes medications, and peripheral neuropathy. Men with recurrent falls were also more likely to have diabetes and use diabetes medications; in addition, men with recurrent falls were more likely to use depression medications, have peripheral neuropathy, and use illicit substances. In an exploratory analysis, we also examined the effect of insulin (versus other diabetes medications or non-diabetes), and diabetes control (HgbA1C ≥ versus <7.5%). Poorly controlled diabetes was associated with a single fall (OR 3.95; 95% CI 1.44, 10.84; p=0.008) among all participants; insulin use (OR 3.59; 95% CI 1.23, 10.48; p=0.019) and controlled diabetes (OR 2.07; 95% CI 1.13, 3.81; p=0.019) were associated with recurrent falls. Other diabetes medications were not significantly associated with falls.
Table 3a.
Characteristics Associated with Increased Odds of Single or Recurrent Falls, Unadjusted, Among Entire Cohort
| Variables | 1 faller/ non-faller vs reference group | OR for 1 faller | P-value | 2+ faller/non-faller vs reference group | OR for 2+ faller | P-value |
|---|---|---|---|---|---|---|
| HIV suppressed (vs negative) | 56/153 vs 83/230 | 1.01(0.68,1.51) | 0.944 | 47/153 vs 66/230 | 1.07(0.7,1.64) | 0.754 |
| HIV with viremia (≥50 vs <50 copies/mL) | 3/12 vs 83/230 | 0.69(0.19,2.52) | 0.577 | 8/12 vs 66/230 | 2.32(0.91,5.92) | 0.077 |
| Age per 5 yrs | NA | 1.03(0.88,1.2) | 0.743 | NA | 1.18(1,1.39) | 0.057 |
| Black (vs white) | 22/65 vs 112/308 | 0.93(0.55,1.58) | 0.791 | 27/65 vs 91/308 | 1.41(0.85,2.33) | 0.187 |
| Other race (vs white) | 8/22 vs 112/308 | 1(0.43,2.31) | 1.000 | 3/22 vs 91/308 | 0.46(0.14,1.58) | 0.217 |
| Chicago (vs Baltimore) | 40/72 vs 37/107 | 1.61(0.94,2.75) | 0.084 | 48/72 vs 13/107 | 5.49(2.77,10.85) | 0.000 |
| Pittsburgh (vs Baltimore) | 31/98 vs 37/107 | 0.91(0.53,1.59) | 0.751 | 44/98 vs 13/107 | 3.69(1.88,7.27) | 0.000 |
| Los Angeles (vs Baltimore) | 34/118 vs 37/107 | 0.83(0.49,1.42) | 0.503 | 16/118 vs 13/107 | 1.12(0.51,2.43) | 0.782 |
| Enrollment after 2001 (vs before 2001) | 25/66 vs 117/329 | 1.07(0.64,1.77) | 0.807 | 31/66 vs 90/329 | 1.72(1.06,2.79) | 0.029 |
| Former smoker (vs non-smoker) | 79/209 vs 47/131 | 1.05(0.69,1.61) | 0.809 | 50/209 vs 42/131 | 0.75(0.47,1.19) | 0.217 |
| Current smoker (vs non-smoker) | 15/53 vs 47/131 | 0.79(0.41,1.53) | 0.483 | 28/53 vs 42/131 | 1.65(0.93,2.93) | 0.089 |
| Heavy alcohol use (yes vs no) | 10/40 vs 131/353 | 0.67(0.33,1.39) | 0.284 | 13/40 vs 107/353 | 1.07(0.55,2.08) | 0.837 |
| Moderate physical activity (vs low) | 32/119 vs 32/77 | 0.65(0.37,1.14) | 0.133 | 34/119 vs 32/77 | 0.69(0.39,1.21) | 0.191 |
| High physical activity (vs low) | 78/199 vs 32/77 | 0.94(0.58,1.54) | 0.814 | 55/199 vs 32/77 | 0.66(0.4,1.11) | 0.116 |
| Overweight (BMI 25–30 kg/m2) (vs normal) | 45/152 vs 63/160 | 0.75(0.48,1.17) | 0.206 | 41/152 vs 58/160 | 0.74(0.47,1.18) | 0.205 |
| Obese (BMI≥ 30 kg/m2) (vs normal) | 34/83 vs 63/160 | 1.04(0.63,1.71) | 0.875 | 22/83 vs 58/160 | 0.73(0.42,1.28) | 0.271 |
| Hypertension (vs no) | 85/238 vs 57/156 | 0.98(0.66,1.45) | 0.909 | 71/238 vs 49/156 | 0.95(0.63,1.44) | 0.808 |
| Diabetes (vs no) | 26/40 vs 115/354 | 2(1.17,3.42) | 0.011 | 22/40 vs 98/354 | 1.99(1.13,3.5) | 0.018 |
| Dyslipidemia (vs no) | 105/310 vs 36/84 | 0.79(0.5,1.24) | 0.304 | 88/310 vs 32/84 | 0.75(0.47,1.19) | 0.221 |
| Liver disease (vs no) | 0/3 vs 142/392 | NA | 1/3 vs 120/392 | 1.09(0.11,10.57) | 0.941 | |
| Renal impairment | 15/38 vs 127/357 | 1.11(0.59,2.09) | 0.747 | 14/38 vs 107/357 | 1.23(0.64,2.35) | 0.534 |
| Hepatitis C infection (vs no) | 7/21 vs 135/374 | 0.92(0.38,2.22) | 0.859 | 7/21 vs 114/374 | 1.09(0.45,2.64) | 0.842 |
| CESD≥16 (vs<16) | 30/64 vs 112/331 | 1.39(0.85,2.25) | 0.187 | 26/64 vs 95/331 | 1.42(0.85,2.36) | 0.182 |
| Diabetes medication (vs no) | 21/34 vs 121/360 | 1.84(1.03,3.29) | 0.040 | 21/34 vs 100/360 | 2.22(1.24,4) | 0.008 |
| Hypertension medication (vs no) | 66/180 vs 76/214 | 1.03(0.7,1.52) | 0.871 | 56/180 vs 65/214 | 1.02(0.68,1.54) | 0.908 |
| Cholesterol lowering medication (vs no ) | 62/193 vs 80/201 | 0.81(0.55,1.19) | 0.277 | 61/193 vs 60/201 | 1.06(0.7,1.59) | 0.783 |
| Depression medication (vs no) | 29/83 vs 113/310 | 0.96(0.6,1.54) | 0.861 | 42/83 vs 79/310 | 1.99(1.27,3.1) | 0.003 |
| Illicit drug (vs no) | 71/162 vs 70/231 | 1.45(0.98,2.13) | 0.061 | 62/162 vs 58/231 | 1.52(1.01,2.3) | 0.044 |
| Stimulants (vs no) | 10/28 vs 131/365 | 1(0.47,2.1) | 0.990 | 14/28 vs 106/365 | 1.72(0.87,3.39) | 0.116 |
| Peripheral neuropathy (vs no) | 45/78 vs 96/314 | 1.89(1.22,2.91) | 0.004 | 41/78 vs 79/314 | 2.09(1.33,3.28) | 0.001 |
| Neurological impairment (vs no) | 7/30 vs 135/362 | 0.63(0.27,1.46) | 0.277 | 12/30 vs 109/362 | 1.33(0.66,2.68) | 0.428 |
Table 3b.
Characteristics Associated with Increased Odds of Single or Recurrent Falls, Unadjusted, Among Men with HIV Only
| Variables | 1 faller/ non-faller vs reference group | OR for 1 faller | P-value | 2+ faller/non-faller vs reference group | OR for 2+ faller | P-value |
|---|---|---|---|---|---|---|
| HIV with viremia (≥50 vs <50 copies/mL) | 3/12 vs 56/153 | 0.68 (0.19, 2.51) | 0.566 | 8/12 vs 47/153 | 2.17 (0.84, 5.63) | 0.111 |
| Age per 5 yrs | NA | 1.11 (0.86, 1.43) | 0.426 | NA | 1.07 (0.82, 1.39) | 0.607 |
| Black (vs white) | 13/40 vs 44/117 | 0.86 (0.42, 1.77) | 0.689 | 14/40 vs 38/117 | 1.08 (0.53, 2.19) | 0.837 |
| Other race (vs white) | 2/8 vs 44/117 | 0.67 (0.14, 3.25) | 0.615 | 3/8 vs 38/117 | 1.15 (0.29, 4.57) | 0.838 |
| Chicago (vs Baltimore) | 21/38 vs 14/45 | 1.78 (0.80, 3.96) | 0.160 | 28/38 vs 5/45 | 6.63 (2.33, 18.9) | <0.001 |
| Pittsburgh (vs Baltimore) | 11/35 vs 14/45 | 1.01 (0.41, 2.50) | 0.982 | 18/35 vs 5/45 | 4.63 (1.56, 13.7) | 0.006 |
| Los Angeles (vs Baltimore) | 13/47 vs 14/45 | 0.89 (0.38,2.10) | 0.788 | 4/47 vs 5/45 | 0.77 (0.19, 3.04) | 0.704 |
| Enrollment after 2001 (vs before 2001) | 13/39 vs 46/126 | 0.91 (0.45,1.86) | 0.802 | 20/39 vs 35/126 | 1.85 (0.96, 3.56) | 0.067 |
| Former smoker (vs non-smoker) | 30/87 vs 19/52 | 0.94 (0.48, 1.84) | 0.865 | 26/87 vs 18/52 | 0.86 (0.43, 1.73) | 0.677 |
| Current smoker (vs non-smoker) | 10/24 vs 19/52 | 1.14 (0.46, 2.82) | 0.776 | 11/24 vs 18/52 | 1.32 (0.54, 3.23) | 0.537 |
| Heavy alcohol use (vs no) | 4/15 vs 55/148 | 0.72 (0.23, 2.26) | 0.570 | 4/15 vs 51/148 | 0.77 (0.25, 2.44) | 0.662 |
| Moderate physical activity (vs low) | 13/44 vs 14/29 | 0.61 (0.25, 1.49) | 0.279 | 18/44 vs 16/29 | 0.74 (0.33, 1.68) | 0.475 |
| High physical activity (vs low) | 32/92 vs 14/29 | 0.72 (0.34, 1.53) | 0.394 | 21/92 vs 16/29 | 0.41 (0.19, 0.90) | 0.025 |
| Overweight (BMI 25–30) (vs normal) | 17/65 vs 32/71 | 0.58 (0.29, 1.14) | 0.116 | 19/65 vs 29/71 | 0.72 (0.37, 1.40) | 0.327 |
| Obese (BMI ≥ 30) (vs normal) | 10/29 vs 32/71 | 0.77 (0.33, 1.76) | 0.528 | 7/29 vs 29/71 | 0.59 (0.23, 1.50) | 0.269 |
| Hypertension (vs no) | 41/99 vs 18/65 | 1.50 (0.79, 2.83) | 0.215 | 37/99 vs 18/65 | 1.35 (0.71, 2.57) | 0.362 |
| Diabetes (vs no) | 11/22 vs 48/142 | 1.48 (0.67, 3.27) | 0.334 | 11/22 vs 44/142 | 1.61 (0.73, 3.59) | 0.240 |
| Dyslipidemia (vs no) | 46/137 vs 13/28 | 0.72 (0.35, 1.51) | 0.389 | 46/137 vs 9/28 | 1.04 (0.46, 2.38) | 0.917 |
| Liver disease (vs no) | 0/1 vs 59/164 | NA | 1/1 vs 54/164 | 3.04 (0.19, 49.4) | 0.435 | |
| eGFR<60 (vs ≥60) | 7/28 vs 52/137 | 0.66 (0.27, 1.60) | 0.357 | 8/28 vs 47/137 | 0.83 (0.35, 1.95) | 0.674 |
| HCV infection (vs no) | 6/11 vs 53/154 | 1.59 (0.56, 4.50) | 0.386 | 5/11 vs 50/154 | 1.40 (0.46, 4.22) | 0.550 |
| CESD≥16 (vs<16) | 18/28 vs 41/137 | 2.15 (1.08, 4.27) | 0.029 | 9/28 vs 46/137 | 0.96 (0.42, 2.18) | 0.917 |
| Diabetes medication (vs no) | 8/19 vs 51/145 | 1.20 (0.49, 2.90) | 0.690 | 11/19 vs 44/145 | 1.91 (0.84, 4.31) | 0.121 |
| Hypertension medication (vs no) | 30/78 vs 29/86 | 1.14 (0.63, 2.07) | 0.665 | 32/78 vs 23/86 | 1.53 (0.83, 2.84) | 0.174 |
| Cholesterol lowering medication (vs no ) | 29/91 vs 30/73 | 0.78 (0.43, 1.41) | 0.403 | 32/91 vs 23/73 | 1.12 (0.60, 2.07) | 0.728 |
| Depression medication (vs no) | 14/52 vs 45/112 | 0.67 (0.34, 1.33) | 0.252 | 20/52 vs 35/112 | 1.23 (0.65, 2.33) | 0.525 |
| Illicit drug (vs no) | 31/71 vs 28/92 | 1.43 (0.79, 2.61) | 0.236 | 33/71 vs 22/92 | 1.94 (1.04, 3.62) | 0.036 |
| Stimulants (vs no) | 7/15 vs 52/148 | 1.33 (0.51, 3.44) | 0.559 | 9/15 vs 46/148 | 1.93 (0.79, 4.70) | 0.148 |
| Peripheral neuropathy (vs no) | 27/45 vs 32/118 | 2.21 (1.19, 4.10) | 0.012 | 2¼5 vs 34/118 | 1.62 (0.85, 3.08) | 0.142 |
| Neurological impairment (vs no) | 4/14 vs 55/149 | 0.77 (0.24, 2.45) | 0.663 | 6/14 vs 49/149 | 1.30 (0.47, 3.58) | 0.607 |
| History of AIDS | 13/28 vs 46/137 | 1.38 (0.66, 2.89) | 0.389 | 7/28 vs 48/137 | 0.71 (0.29, 1.74) | 0.458 |
| CD4<200 (vs ≥500) cells/uL | 1/6 vs 42/119 | 0.47 (0.06, 4.04) | 0.493 | 0/6 vs 42/119 | NA | 0.982 |
| CD4 200–499 (vs ≥500) cells/uL | 16/37 vs 42/119 | 1.23 (0.62, 2.43) | 0.560 | 13/37 vs 42/119 | 1.00 (0.48, 2.05) | 0.990 |
| Nadir CD4<200 (vs ≥500) cells/uL | 17/66 vs 7/18 | 0.66 (0.24, 1.84) | 0.430 | 18/66 vs 5/18 | 0.98 (0.32, 3.01) | 0.974 |
| Nadir CD4 200–499 (vs ≥500) cells/uL | 35/81 vs 7/18 | 1.11 (0.43, 2.90) | 0.829 | 30/81 vs 5/18 | 1.33 (0.45, 3.91) | 0.600 |
| Current efavirenz use (vs no) | 8/12 vs 51/150 | 1.96 (0.76, 5.07) | 0.164 | 10/12 vs 45/150 | 2.78 (1.13, 6.85) | 0.027 |
| Ever stavudine use (vs no) | 41/94 vs 18/68 | 1.65 (0.87, 3.11) | 0.124 | 29/94 vs 26/68 | 0.81 (0.44, 1.49) | 0.494 |
| Ever didanosine use (vs no) | 30/64 vs 29/98 | 1.58 (0.87, 2.89) | 0.133 | 20/64 vs 35/98 | 0.87 (0.46, 1.65) | 0.679 |
| Current integrase use (vs no) | 15/43 vs 44/119 | 0.94 (0.48, 1.87) | 0.867 | 12/43 vs 43/119 | 0.77 (0.37, 1.60) | 0.487 |
| Current protein inhibitor use (vs no) | 25/86 vs 34/76 | 0.65 (0.36, 1.19) | 0.160 | 26/86 vs 29/76 | 0.79 (0.43, 1.46) | 0.456 |
| On HAART (vs no) | 53/155 vs 6/7 | 0.40 (0.13, 1.24) | 0.112 | 53/155 vs 2/7 | 1.20 (0.24, 5.94) | 0.826 |
| Duration of any protease inhibitor use | NA | 0.99 (0.94, 1.04) | 0.754 | NA | 1.00 (0.95, 1.05) | 0.900 |
| Duration of HAART | NA | 1.00 (0.93, 1.07) | 0.937 | NA | 1.02 (0.95, 1.09) | 0.607 |
HAART, highly active antiretroviral therapy
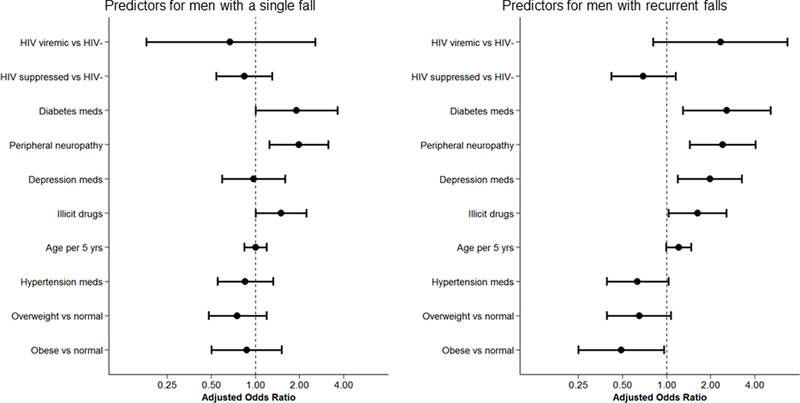
In multivariate models (Figures 1a and 1b), the odds of experiencing a single fall remained greater among men taking diabetes medication and with peripheral neuropathy. The odds of experiencing recurrent falls were greater among men reporting illicit drug use, taking diabetes or depression medications, and with peripheral neuropathy, while obesity was protective. The effect of diabetes medication with a high-risk for hypoglycemia (insulin and sulfonylureas) was greater than that of other lower-risk diabetes medications (OR 5.78; 95% CI 1.49, 22.43; p=0.01 vs OR 1.34; 95% CI 0.14, 12.8; p=0.79). In separate multivariate sensitivity analyses, poorly controlled diabetes was associated with a single fall (OR 3.75; 95% CI 1.27, 11.11; p=0.017) and insulin use was associated with recurrent falls (OR 4.00; 95% CI 1.20, 13.38; 0.024).
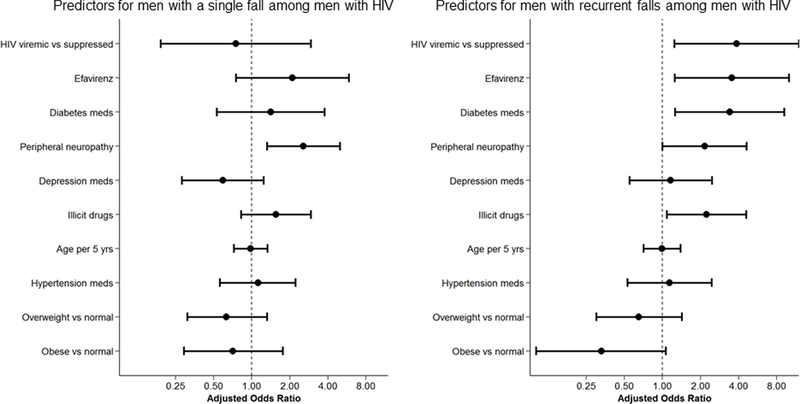
Figure 1.
Predictors of being a single or recurrent faller from multinomial logistic regression in the entire cohort (1a) or among men with HIV only (1b).
In univariate models restricted to PLWH, odds of a single fall were greater among men with depressive symptoms and peripheral neuropathy; odds of experiencing recurrent falls differed by study site, and was greater among those with lower physical activity, with illicit substance use, and efavirenz use. In separate sensitivity analyses, insulin was associated with recurrent falls (OR 5.49; 1.26, 23.91; p=0.023); other diabetes medications and diabetes control were not associated with single or recurrent falls. In adjusted models restricted to PLWH, only peripheral neuropathy was associated with a single fall; detectable HIV-1 RNA, use of efavirenz or diabetes medications, illicit drug use, and peripheral neuropathy were associated with a greater odds of experiencing recurrent falls. In separate sensitivity analyses, insulin remained associated with a significantly greater risk of recurrent falls (OR 7.40; 95% CI 1.37, 39.98; p=0.020).
To further investigate the HIV-1 RNA findings, we compared men with lower versus higher (>75th percentile) HIV-1 RNA copy-years, corresponding to log10(viral copy-years) of 2.07, or 119 copy-years/mL. PLWH in the upper quartile of HIV-1 RNA remained at significantly increased odds of recurrent falls (OR 2.70; 95% CI 1.22, 5.99, p= 0.014), although the effect was attenuated. To evaluate the effect of low-level of HIV-1 viremia, we next excluded participants with a baseline HIV-1 RNA > 500 copies/mL (n=4). The odds of recurrent falls among PLWH and low level baseline HIV-1 viremia (50–500 copies/mL) remained significant (OR 5.45; 95% CI 1.6, 18.5; p=0.007). Lastly, we assessed the effect of any subsequent HIV-1 RNA above detection over the 2-year study follow-up period (n=93). In contrast to the association with viral copy years and baseline HIV-RNA, the odds of recurrent falls with any detectable HIV-1 RNA during the study follow-up was no longer significant (OR 1.89; 95% CI 0.88, 4.05, p=0.101).
Lastly, we investigated the impact of medications often associated with falls (anxiolytics and opioids) and the effect of polypharmacy on the risk of single and recurrent falls when added to the current models. Seven percent of men with HIV and 5% of men without HIV utilized pain medications, 33% of men with and 29% of men without HIV used anxiolytics, with 61% of the men with and 58% of men without HIV taking 5 or more non-ART medications. In the univariate analysis for all men, polypharmacy (5 or more non-ART medications) was associated with recurrent falls (OR=1.79, P=0.008); this association was no longer seen in the fully adjusted model (OR=1.53, P=0.127). The association with pain medications or anxiolytics was not significant in univariate models. Among men with HIV, polypharmacy, anxiolytics, and opioids were not significantly associated with falls in univariate models.
Characteristics Associated with Fall-Related Injury or Fracture
Among all men, the odds of having an injurious fall was greater among men with peripheral neuropathy (OR 2.12 [2.09, 4.12]. p=0.026). In models restricted to PLWH, current efavirenz use was associated with increased odds of having an injurious fall (OR 5.44 [1.36, 21.8], p=0.003) while longer duration of ART (OR 0.41 [0.23, 0.74], p=0.014) was protective; peripheral neuropathy (OR 2.61 [0.85, 8.01], p=0.093) and detectable HIV-1 RNA (3.61 [0.80, 16.38], p=0.096) were non-significantly associated with a higher risk of an injurious fall. Detectable HIV-1 RNA (OR 4.48, [0.77, 25.99], p=0.094) and diabetes medications (OR 3.19 [0.94, 10.88], p=0.064) were also associated with increased risk of having a fall resulting in fracture, but did not reach statistical significance; higher self-reported physical activity was the only factor independently associated with a protective effect on risk of having a fall with a fracture (OR 0.23, [0.08, 0.72], p=0.011).
Physical Function and Balance in Fall Risk
Lower balance confidence on the ABC survey was associated with recurrent falls; the strength of this association was attenuated but similar with the ABC-6 survey. Slower time to complete 10 chair rises was the only objective physical function measure associated with recurrent falls (Table 4).
Table 4.
Physical Function and Balance Characteristics Associated with Fall Risk
| Characteristic | Odds (95% CI) for 1 Faller | P-value | Odds (95% CI) for 2+ Faller | P-value |
|---|---|---|---|---|
| ABC ≤ 80 | 1.67(0.82,3.39) | 0.156 | 2.21(1.07,4.54) | 0.032 |
| ABC ≤ 90 | 1.62(1.01,2.58) | 0.045 | 1.95(1.19,3.18) | 0.008 |
| ABC-6 ≤ 80 | 1.56(0.96,2.56) | 0.075 | 1.94(1.15,3.26) | 0.012 |
| ABC-6 ≤ 90 | 1.38(0.92,2.08) | 0.117 | 1.59(1.01,2.48) | 0.044 |
| Tandem Stand | 1.21(0.8,1.83) | 0.367 | 0.99(0.63,1.56) | 0.957 |
| Functional Reach | 1.07(0.84,1.35) | 0.594 | 0.98(0.76,1.26) | 0.859 |
| 10-time chair rise | 1.15(0.94,1.42) | 0.177 | 1.26(1,1.59) | 0.047 |
| Grip Strength | 0.99(0.81,1.21) | 0.903 | 1(0.8,1.25) | 0.982 |
| Gait speed | 0.97(0.79,1.21) | 0.809 | 1.06(0.82,1.36) | 0.652 |
| SPPB Score <10 | 1.25(0.71,2.2) | 0.434 | 1.22(0.64,2.32) | 0.546 |
| Pre-frailty | 1.13(0.73,1.73) | 0.591 | 1.5(0.94,2.4) | 0.093 |
| Frailty | 1.35(0.54,3.35) | 0.521 | 1.23(0.43,3.55) | 0.698 |
ABC, activities-specific balance confidence; SPPB, short physical performance battery
Discussion
In this prospective study of falls among older (ages 50–79) PLWH and men without HIV, nearly half of men sustained at least one fall over 2 years, with approximately 10% of falls leading to injury and almost 5% resulting in a fracture. Similar to prior findings from demographically comparable populations, we did not find that PLWH were more likely to fall than men without HIV. We did however detect a significant association of recurrent falls with detectable baseline HIV-1 RNA or greater burden of HIV-1 RNA over the study period. Consonant with these associations is the protective effect seen with longer duration of ART on injurious falls. Not surprisingly with the well-established link to neurological side effects including dizziness30, efavirenz use was associated with an increased risk of falls. Although we are limited in our ability to draw conclusions from our findings regarding incomplete HIV-1 RNA suppression and fall risk because of the small number of men with detectable HIV-1 RNA, our results support a growing body of literature underscoring the importance of consistent virologic suppression to decrease risk for multiple non-HIV outcomes31.
Similar to prior studies in populations with and without HIV, other fall risk factors included neuropathy, use of diabetes and depression medications, and illicit substance use4,11,32. The association between diabetes and fall or fracture risk is likely multifactorial: older adults with diabetes may have neuropathy, poor muscle quality, and multimorbidity. Further, fall risk tended to be greatest with use of diabetes medications with the highest potential for hypoglycemia. The extent to which risk for falls is directly attributable to diabetes, medications, and intensity of diabetes management, however, is difficult to parse. In a meta-analysis of nearly 15,000 older adults without HIV, diabetes was associated with increased falls (relative risk 1.64), but the risk was primarily seen among insulin-treated vs non-insulin treated adults (RR 1.94 vs 1.27)33. Furthermore, intensive management of blood glucose and blood pressure among older adults with diabetes did not increase fall risk in the ACCORD trial34,35. In summary, although diabetes may be a risk for falls, the existing literature does not suggest that less aggressive glucose control lowers fall risk.
Associations between falls and physical activity, slow chair rise time, and poor balance confidence highlight potentially modifiable risks for improvement of fall risk in our population. Both chair stand time and poor balance confidence or fear of falling were strong predictors of falls, are easy to assess in the clinical setting, and are amenable to improvement with exercise. In contrast to prior studies4,5, we surprisingly did not find an association between falls and other measures of physical function including balance impairment despite the relatively high portion of participants with difficulties by tandem stand (45% of men with HIV). Perhaps our men were more fit, as suggested by a similar proportion of SPPB impairment (13% with SPPB <10) despite a median age that was 10 years younger10. The US Preventive Services Task Force and other fall reduction programs emphasize exercise interventions to prevent falls among older at-risk adults, with additional interventions based on clinical judgement36,37–39,40. The best type of exercise for fall reduction among older PLWH has not been investigated.
Our detailed falls reporting tool resulted in a few additional notable findings. First, PLWH were more likely to report a fall in relation to a pet. The CDC previously reported that pet-related injuries most commonly involved dogs, were described as tripping over or being pulled by the dog (52%), and most commonly occurred while walking a dog (26%)41. Thirty percent of both dog- and cat-related injuries were fractures, and the highest rates of fractures occurred among older adults. As our finding was a post-hoc observation, we did not collect information on pet ownership and were unable to determine if PLWH were simply more likely to own a pet. We also noted high rates of falls associated with steps/curbs and snow/ice. Evaluation for impairment in visual depth perception in addition to counseling regarding safety around pets, built and natural environmental factors, appropriate footwear in the winter, lighting in home stairwells, and avoidance of distractions while walking could be incorporated into routine falls assessment for older adults both with and without HIV1,42.
Several limitations of the present study should be acknowledged. First, the study population was restricted to men with or at risk for HIV. Second, men in the MACS have been routinely engaged in study participation and may be more fit and adherent than other populations, which may influence fall risk factors. Third, sites differed in fall reporting, which may have been due to differences in climate, participant briefing, or other characteristics. To control for this, all multivariate analyses adjusted for site. The large cohort of older men with a comparable HIV-uninfected control group, the use of a prospective reporting tool, ascertainment of fall risk factors prior to reported falls, and the detailed description of factors contributing to each fall are clear strengths.
In summary, the rate of falls, recurrent falls, and falls with fracture were high among older men with or at risk for HIV infection. Our novel finding that HIV-1 viremia was associated with fall risk, after accounting for common fall risk factors, further emphasizes the importance of ART adherence in non-HIV comorbidity management. Several additional factors may help identify persons at risk for falls (diabetes, neuropathy, illicit substance use) or potential targets for interventions (increasing physical activity, improving physical function, transitioning off efavirenz, and counseling regarding pets, curbs, and other potential hazards). Finally, physical activity was the only factor that was associated with (and protective for) falls complicated by fracture. Extrapolating from these findings and our prior work, we suggest that fall risk reduction among older PLWH should mirror those of the US Preventative Task Force1 with a focus on physical activity and additional risk reduction maximized through ART adherence and transition to use of newer non-efavirenz-containing ART regimens.
Acknowledgements
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick, Todd Brown), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza, Otto Yang), U01-AI35040; University of Pittsburgh (Charles Rinaldo, Lawrence Kingsley, Jeremy Martinson), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D’Souza), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Funding: The MACS Bone Strength Substudy (BOSS) was supported by NIH (NIAID) R01AI095089 (TTB). The Multicenter AIDS Cohort Study is supported by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). Additional support by the National Heart, Lung, and Blood Institute, the National Institute of Immunology, Allergy, and Infectious Disease and the National Institute of Aging of the National Institutes of Health under award numbers K24 AI120834 to TTB; NIA K23 AG050260 and R01AG054366 to KME; K23 AI110532 to JEL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Grossman DC, Curry SJ, Owens DK, et al. Interventions to Prevent Falls in Community-Dwelling Older Adults: US Preventive Services Task Force Recommendation Statement. Jama 2018; 319(16): 1696–704. [DOI] [PubMed] [Google Scholar]
- 2.Auais M, Alvarado B, Guerra R, et al. Fear of falling and its association with life-space mobility of older adults: a cross-sectional analysis using data from five international sites. Age Ageing 2017; 46(3): 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 1997; 337(18): 1279–84. [DOI] [PubMed] [Google Scholar]
- 4.Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr 2012; 61(4): 484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassiopoulos K, Abdo M, Wu K, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults: a prospective cohort study. Aids 2017. [DOI] [PMC free article] [PubMed]
- 6.McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E. A Pharmacist-Led Program to Evaluate and Reduce Polypharmacy and Potentially Inappropriate Prescribing in Older HIV-Positive Patients. Pharmacotherapy 2017; 37(12): 1498–506. [DOI] [PubMed] [Google Scholar]
- 7.Althoff KN, Jacobson LP, Cranston RD, et al. Age, Comorbidities, and AIDS Predict a Frailty Phenotype in Men Who Have Sex With Men. J Gerontol A Biol Sci Med Sci 2013. [DOI] [PMC free article] [PubMed]
- 8.John MD, Greene M, Hessol NA, et al. Geriatric Assessments and Association With VACS Index Among HIV-Infected Older Adults in San Francisco. J Acquir Immune Defic Syndr 2016; 72(5): 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Hoover DR, Shi Q, et al. Longitudinal study of falls among HIV-infected and uninfected women: the role of cognition. Antivir Ther 2017. [DOI] [PMC free article] [PubMed]
- 10.Erlandson KM, Plankey MW, Springer G, et al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med 2016; 17(10): 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Hoover DR, Shi Q, et al. Falls among middle-aged women in the Women’s Interagency HIV Study. Antivir Ther 2016; 21(8): 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapp K, Freiberger E, Todd C, et al. Fall incidence in Germany: results of two population-based studies, and comparison of retrospective and prospective falls data collection methods. BMC Geriatr 2014; 14: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satariano WA, Wang C, Kealey ME, Kurtovich E, Phelan EA. Risk Profiles for Falls among Older Adults: New Directions for Prevention. Frontiers in public health 2017; 5: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsey JL, Berry SD, Procter-Gray E, et al. Indoor and outdoor falls in older adults are different: the maintenance of balance, independent living, intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc 2010; 58(11): 2135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013; 63(2): 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant PM, Kitch D, McComsey GA, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. Aids 2016; 30(18): 2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins KL, Zhang L, Ng DK, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. Aids 2018; 32(10): 1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001; 56(10): M644–9. [DOI] [PubMed] [Google Scholar]
- 19.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol 1990; 45(6): M192–7. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3): M146–56. [DOI] [PubMed] [Google Scholar]
- 21.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995; 50A(1): M28–34. [DOI] [PubMed] [Google Scholar]
- 22.Schepens S, Goldberg A, Wallace M. The short version of the Activities-specific Balance Confidence (ABC) scale: its validity, reliability, and relationship to balance impairment and falls in older adults. Archives of gerontology and geriatrics 2010; 51(1): 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1998; 53(4): M287–94. [DOI] [PubMed] [Google Scholar]
- 24.Portegijs E, Edgren J, Salpakoski A, et al. Balance confidence was associated with mobility and balance performance in older people with fall-related hip fracture: a cross-sectional study. Arch Phys Med Rehabil 2012; 93(12): 2340–6. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130(6): 461–70. [DOI] [PubMed] [Google Scholar]
- 26.Sacktor N, Skolasky RL, Cox C, et al. Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: impact of age and serostatus. J Neurovirol 2010; 16(5): 335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anziska Y, Helzner EP, Crystal H, et al. The relationship between race and HIV-distal sensory polyneuropathy in a large cohort of US women. Journal of the neurological sciences 2012; 315(1–2): 129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Haberlen SA, Palella FJ Jr., et al. Viremia copy-years and mortality among cART-initiating HIV-positive individuals: how much viral load history is enough? Aids 2018. [DOI] [PMC free article] [PubMed]
- 29.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35(8): 1381–95. [DOI] [PubMed] [Google Scholar]
- 30.Seden K, Kiiza D, Laker E, et al. High prevalence and long duration of nervous system and psychiatric adverse drug reactions in Ugandan patients taking efavirenz 600 mg daily. J Antimicrob Chemother 2018; 73(11): 3158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis 2016; 63(12): 1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Hoover DR, Shi Q, et al. Longitudinal study of falls among HIV-infected and uninfected women: the role of cognition. Antivir Ther 2018; 23(2): 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing 2016; 45(6): 761–7. [DOI] [PubMed] [Google Scholar]
- 34.Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med 2014; 29(12): 1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes care 2012; 35(7): 1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2018; 319(16): 1705–16. [DOI] [PubMed] [Google Scholar]
- 37.Shubert TE, Smith ML, Jiang L, Ory MG. Disseminating the Otago Exercise Program in the United States: Perceived and Actual Physical Performance Improvements From Participants. J Appl Gerontol 2018; 37(1): 79–98. [DOI] [PubMed] [Google Scholar]
- 38.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. Bmj 1997; 315(7115): 1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shubert TE, Smith ML, Goto L, Jiang L, Ory MG. Otago Exercise Program in the United States: Comparison of 2 Implementation Models. Phys Ther 2017; 97(2): 187–97. [DOI] [PubMed] [Google Scholar]
- 40.Morrison S, Simmons R, Colberg SR, Parson HK, Vinik AI. Supervised Balance Training and Wii Fit–Based Exercises Lower Falls Risk in Older Adults With Type 2 Diabetes. Journal of the American Medical Directors Association 2018; 19(2): 185.e7-.e13. [DOI] [PubMed] [Google Scholar]
- 41.Nonfatal fall-related injuries associated with dogs and cats--United States, 2001–2006. MMWR Morbidity and mortality weekly report 2009; 58(11): 277–81. [PubMed] [Google Scholar]
- 42.Bouxsein ML, Szulc P, Munoz F, Thrall E, Sornay-Rendu E, Delmas PD. Contribution of trochanteric soft tissues to fall force estimates, the factor of risk, and prediction of hip fracture risk. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2007; 22(6): 825–31. [DOI] [PubMed] [Google Scholar]