Figure 1. The conformational regulation of proteins and their engineered analogs.
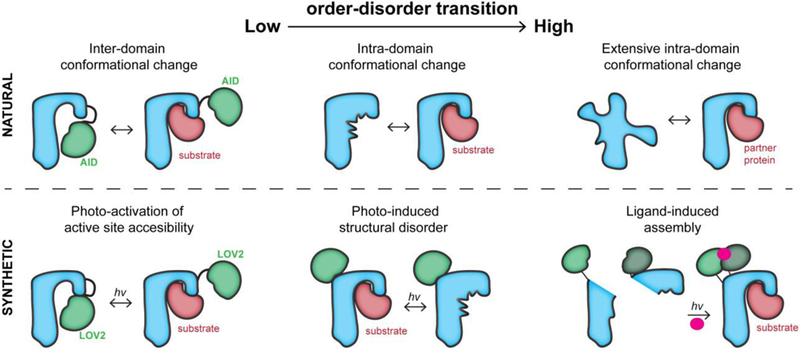
Top, left: An auto-inhibitory domain (AID) blocks the active site in the inactive state. Upon post-translational modification or protein binding, the AID undocks from the active site. Top, center: The active site undergoes a disorder-order transition during activation, enabling binding. Top, right: An intrinsically disordered protein becomes ordered upon binding to a partner protein. Bottom, left: The light-sensitive domain LOV2 blocks the active site. Upon irradiation, it undocks from the active site. Bottom, center: The LOV2 domain inserted into a loop of the host protein undergoes a light-induced conformational change. This distorts the active site allosterically. Bottom, right; The split halves of a protein reassemble upon binding a small molecule that enables dimerization. The small molecule is activated through cleavage of a light-sensitive protecting group.
