Figure 1.
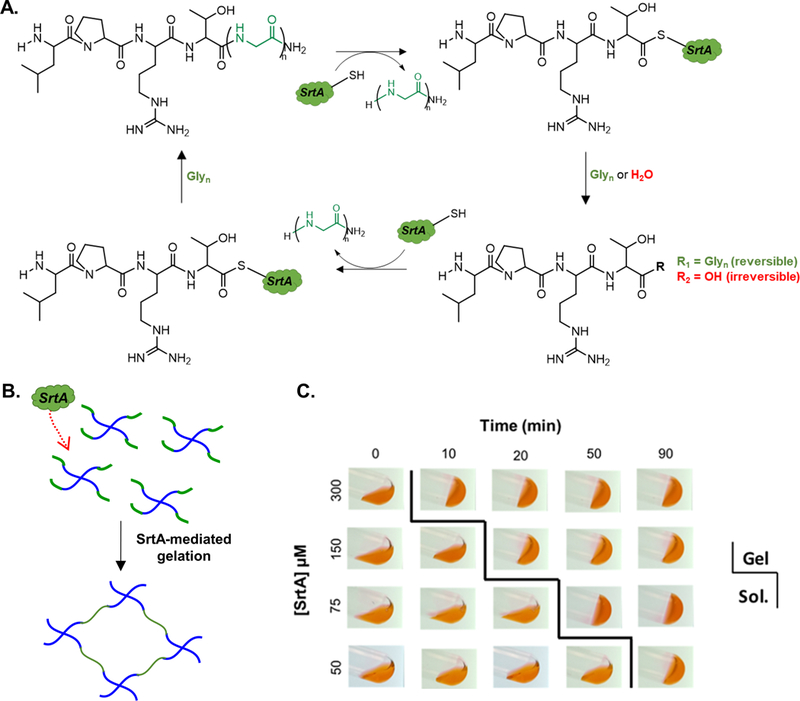
(A) Schematic of reversible SrtA-transpeptidation reaction. SrtA cleaves amide bond between threonine and glycine residues of LPRTG substrate and forms a thioacyl intermediate. Nucleophilic oligoglycine or water resolve intermediate. Note that glycine-containing product, LPRT(G)n, can undergo multiple cycles of transpeptidation with additional incubation with SrtA and oligoglycine. (B) SrtA-mediated gelation of PEG-peptide hydrogels. SrtA was used to initiate crosslinking between PEG-peptide conjugates. Cysteine containing SrtA substrates (i.e., CLPRTG and GGGGC) were conjugated to PEG-NB in the presence of UV light and photoinitiator LAP. (C) Test tube tilt test to track timing of sol-gel transition using 6 wt% PEG8NB-peptide conjugates, RGGGG:LPRTG=2). Eosin-Y (1 mM, red dye) was added for image clarity.
