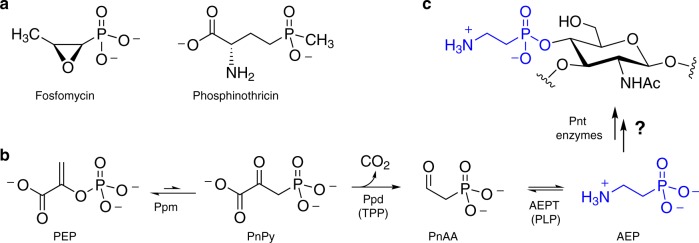
Fig. 1.
Phosphonates in nature. a Small molecule bioactive natural products fosfomycin and phosphinothricin. b Biosynthesis of the common phosphonate AEP from phosphoenolpyruvate (PEP) via the intermediacy of phosphonopyruvate (PnPy) and phosphonoacetaldehyde (PnAA). Cofactors in parentheses are thiamine pyrophosphate (TPP) and pyridoxal 5′-phosphate (PLP). Unknown phosphonyl tailoring (Pnt) biosynthetic steps lead from AEP to c, cell surface phosphonoglycans like that isolated from Bacteroides fragilis