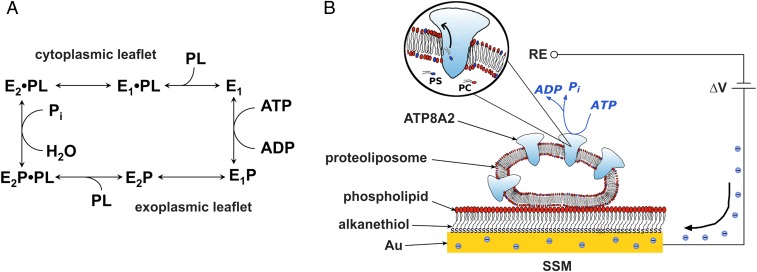
Fig. 1.
Simplified scheme of the ATP8A2 reaction cycle and schematic diagram of the SSM method. (A) The scheme is based on functional similarities to the ion-transporting P2-ATPases (5, 6). E1, E1P, E2P, and E2 are different enzyme conformational states, “P” indicates covalently bound phosphate. The phospholipid substrate, PL (PS or PE), enters the cycle from the exoplasmic leaflet of the lipid bilayer by binding to the E2P phosphoenzyme, thereby stimulating the dephosphorylation and release of the lipid substrate toward the cytoplasmic leaflet as consequence of the E2 to E1 conformational change. (B) Reconstituted proteoliposome containing ATP8A2 adsorbed on the SSM surface and subjected to an ATP concentration jump (not drawn to scale). If the ATP concentration jump induces net charge displacement across the ATPase, a current signal is detected along the external circuit (the blue spheres represent electrons) to keep constant the potential difference (ΔV) applied across the whole system. RE, reference electrode.