Abstract
Background
Newcastle disease virus (NDV) is a dangerous viral disease, infecting a broad range of birds, and has a fatal effect on the poultry industries. The attachment and consequently fusion of the virus to the host cell membrane is directed by the two superficial glycoproteins, the hemagglutinin-neuraminidase (HN) and the fusion (F) which is considered as the important targets for the poultry immune response.
Objectives
The principal goal of this investigation was to realize the potential efficacy of the E. coli expression system for the production of the multi-epitopic HN, and F proteins with respect to the ability for the stimulation of the immune system and production of the cross-reactive antibodies in mice.
Materials and Methods
The recombinant HN and F (rHN, rF) have accumulated almost 40% of the total bacterial proteins. The presence of rHN and rF proteins recognized by the Western blotting with specific anti-HN, anti-F, anti-Newcastle B1, and anti-poly 6x His-tag antibodies. Furthermore, both rHN and rF have shown the specific reactivity against the Newcastle B1 antiserum as a standard strain.
Results
The ELISA analysis showed that the higher dilutions of the antibody against Newcastle B1 could react with the as least quantity as 100 ng of the purified rHN, and rF. Cross-reactivity analysis of the sera from the mice immunized with Newcastle B1 in two time points indicated that the raise of anti-Newcastle B1, anti-HN and anti-F antibodies peaked at 28 days post immunization (dpi). Moreover, temporal variation in IgG titration between both time points was significant at 5% probability level.
Conclusion
The results provided valuable information about the cross-reactivity patterns and biological activity of the multi-epitopic proteins compared to the NDV standard strain which was determined by the Western blotting and ELISA.
Keywords: Affinity purification, Fusion, Hemagglutinin-neuraminidase, Immunization, Newcastle disease virus
1. Background
Newcastle disease (ND) is a highly contagious viral disease in the domesticated birds, especially chicken, which causes a large economic loss in the poultry industry (1). Because of this impact, the World Organization for Animal Health (OIE) has considered this viral disease as the most significant disease of poultry (2). The causative agent of the infection is an RNA virus that belongs to the avian paramyxovirus serotype 1 (APMV-1), which shows variable pathogenicity and severity based on the strain’s virulence (3). The infection of the host cells by the Newcastle disease virus (NDV) is accomplished through the interaction of the two major surface molecules, hemagglutinin-neuraminidase (HN) and fusion glycoproteins (F) (4). The accurate mechanism of the viral membrane fusion and infection process has been investigated by many researchers in-depth (5-7). Briefly, the HN is responsible for attaching to the cell surface receptors including sialic acid and then F molecule triggers the fusion process. The insertion into the host cell membrane is processed by the F protein which plays a crucial role in the NDV pathogenicity (8). The immunological analysis has shown that both HN and F proteins could act as antigens in the virus structure and the neutralizing antibodies could be produced against these proteins in the infected animal (9, 10).
The live attenuated vaccines offer the possibility of the long-term protection, can influence the immune system to generate memory cells, and elicit good immunological responses. If a vaccine can evoke an incomplete immune response, it may be considered successful for preventing disease (11). Vaccines which employ live attenuated viruses are associated with some risks including the potential to induce disease, reversion to the virulence, as well as environmental spread. Also nowadays, most of the vaccines developed for the research application consist of the purified recombinant proteins or microbe’s subunits (12). One of the major problems with this kind of vaccines is the selection of the correct antigen or antigens (13). The multimeric proteins which are connected by peptide linker could generate a broad range of immune responses and increase the immunogenicity of the recombinant proteins (14).
To achieve a more effective control of NDV, the early diagnosis is a critical point. Although the gold standard for the detection of NDV is hemagglutinin-inhibition (HI) test, in case of a high number of the serum samples, the ELISA test is the most reliable method. The immunodiagnostic methods such as ELISA have provided a high sensitivity and efficacy for the detection of NDV specific immune responses (15). For example, the expression of the recombinant ectodomain (nt 142-1739) of the HN protein as inclusion bodies in the E. coli and its immunoblotting analyses have revealed the possibility of this recombinant protein as an antigen for the diagnosis of ND in the poultry (16). However, there is relatively limited information about the immunological properties of the recombinant surface glycoproteins of the NDV expressed in the E. coli system. There are a few reports about the successful expression and purification of NDV glycoproteins in the E. coli in which the recombinant HN was used as main the component in the ELISA based method (17) and purification of the immunogenic F protein that were studied elsewhere (18, 19).
An efficient production of the recombinant proteins in a highly purified and the well-characterized form could be achieved the through application of different expression systems such as E. coli (20). For example, numerous viral glycoproteins used in the immunological studies were produced in the E. coli such as: non-structural protein-1 (NS1) from the influenza virus (21), NS1 protein of the dengue virus type 2 (22), the glycoprotein E2 of the swine fever virus (23), and glycoprotein gp160 of the Human Immunodeficiency Virus type 1 (HIV1) (24). Therefore, a simple and efficient method for antigens preparation could be used to evaluate vaccine efficiency and detection of the NDV infection.
2. Objectives
Multi-domain biological molecules which consist of several fused DNA fragments can present multiple functional properties resulting from each of its domains and subsequently induce a more effective response (25). Hence, in the present study, a chimeric HN-F construct which has already been designed by in silico assembling potential and conserved epitopic regions of the HN and F proteins of the NDV was used for further immunological analyses (26). To compare the expression efficiency, we attempted to express two multi-domain HN and F proteins individually in the E. coli. Furthermore, different factors such as solubility of the recombinant proteins after production in the heterologous host, purification process facility, immune system stimulation, production of the cross-reactive immune sera, and finally the utility of these specific immune sera to immunodiagnosis of the NDV were analyzed.
3. Materials and Methods
3.1. Plasmids, Bacterial Strains, and Media
PET-32a (+) plasmid (Novagen, USA), Rosetta-gami B (DE3) and DH5α strains (Pasteur Institute of Iran) were used in this study. The Luria-Bertani (LB) broth or LB agar supplemented with the antibiotic (ampicillin, 100 μg.mL-1, Sigma) was used for routine bacterial culture.
3.2. Recombinant HN and F Plasmids Construction and Expression
The single peptide construction consisted of the antigenic domains of HN and F glycoproteins was used in this study (26). Briefly, the epitopic amino acids from both HN (head and stalk domains) and F (HRA and HRB regions) proteins were selected and connected together using a linker consisting of several highly hydrophobic amino acids. The hn (1000 bp) and f (852 bp) synthetic gene were amplified from the synthetic HN-F gene (Gene Bank accession no. JX442483) using the specific primers (Table 1). For further cloning procedure, the BamHI and XhoI restriction sites were introduced at the 5′ terminus of the each forward and reverse primers, respectively. PCR amplification using DNA polymerase was performed for 30 cycles of 1 min at 94 °C, 1 min at 60 °C, and 2 min at 72 °C followed by the final extension at 72 °C for 10 min. The PCR products were cloned into the expression vector pET-32a (+), downstream of the T7 promoter, the thioredoxin (Trx-tag), and the His-tag sequences. The construct was transformed into the E. coli Rosetta-gami B (DE3) competent cell. The presence of the each gene in the expression host was verified by the PCR, digestion with the restriction endonucleases, and finally by sequencing.
Table 1.
Primer sequences used in this experiment
| Primer | Sequence (5′-3′) | Restriction site | PCR product (length; bp) |
|---|---|---|---|
| HNf | TCTAAGGATCCACACCACTGGGTTGCGACA | BamHI | hn (999) |
| HNr | CTTGCTCTCGAGTTATTCTGTCTTCAGGAGTGCCA | XhoI | |
| Ff | TGTTCGGATCCAAAAACATCTCCATCCAAGA | BamHI | f (852) |
| Fr | CTATTGCTCGAGTTATGAATCGTACAGGATTGGGT | XhoI |
3.3. Expression and Purification of the Recombinant Proteins
E. coli Rosetta-gami strain carrying the recombinant plasmid of pET-32a: HN and pET-32a: F were grown at 37 °C to an OD600 of 0.6. The culture was induced by 1 mM isopropyl-β-D-galactopyranoside (IPTG, Sigma) and incubated at 37 °C for further 4 hours. Cells were harvested by centrifugation (5000 ×g, 10 min, 25 οC) and resuspended in the lysis buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH 8.0). After sonication (6 times for 10 second with high power), the lysate was centrifuged (15 min, 10000 ×g, 4 °C) and the supernatant was applied on a Ni-NTA chromatography column (Qiagen, USA). The two recombinant proteins appeared in inclusion bodies after analysis on 12% SDS-PAGE. The denaturing condition was used for purification of the recombinant HN and F. The purification was performed based on the procedure described by the manufacturer with minor changes. The lysis buffer was used for equilibration of the Nickel-nitrilotriacetic acid (Ni-NTA) column. The protein solution was loaded precisely onto the column (flow rate of 0.5 mL.min-1). The impurity was removed two times by washing the column with the washing buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea) with different pH (i.e., pH: 6.3 and pH 5.9) for HN and (pH 7.0 and pH 6.3) for F proteins, respectively. The recombinant proteins were eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea) at pH 4.5. The protein concentration was determined by the Bradford method with BSA (bovine serum albumin) as a standard. Finally, a stepwise dialysis was performed until urea reached a concentration of zero M and then repeated two times against PBS buffer (pH: 7.4) containing 10% glycerol and subsequently without glycerol to prevent protein aggregation.
3.4. Animal Model Immunization
To determine the antigenicity of the recombinant HN and F proteins, twenty Balb/c mice (female, 6-8-weeks-old, Pasteur Institute, Tehran, Iran) were randomly divided into four groups (A to D) and acclimatized for 1 week. Group A and B were respectively inoculated with 15 μg of the purified rHN and rF subcutaneously into the quadriceps muscle using complete Freund’s adjuvant (CFA, Sigma, USA). The group C was immunized with a commercial vaccine (B1 strain) as the positive control (a generous gift from Dr. M. M. Ebrahimi from Razi Vaccine and Serum Research Institute, Karaj, Iran) and the last group (D) was immunized with PBS plus CFA as the negative control. The immunization continued by two booster doses, each 10 μg of the recombinant antigens with the incomplete Freund’s adjuvant (IFA, Sigma, USA) and finally one booster (2 μg) via intraperitoneal route at an interval of two weeks. The immune serum was prepared from the blood sample of each mice group. For serum preparation, the animal’s blood was transferred into vials and allowed to clot for 30 min, the serum was collected by centrifugation, and was kept frozen at -70 °C until use. The serum samples of each mice group were prepared and then pooled for the immunological analyses.
3.5. Western Blotting
The recombinant proteins (rHN and rF) were separated by 12% SDS-PAGE and transferred onto PVDF membrane (Roche, Germany). The membrane blocking was done with non-fat skim milk (5%) in TBS buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.5) containing 0.05% Tween 20 at 37 °C for 2 hours. The membrane was further incubated with antiserum against rHN and rF from the immunized mice (1:1000 dilution) and hyperimmune serum specific to Newcastle B1 strain (1:500), separately. The HRP-conjugated goat anti-mouse IgG (1:2000; Sigma, Germany) was used as the secondary antibody. Finally, the membrane was soaked in 3, 3′-Diaminobenzidine tablet (DAB Reagents; Sigma) for signal development. This procedure was repeated with HRP-conjugated anti-His tag (1:500, Roche) for both recombinant HN and F proteins. Furthermore, to verify the cross-reactivity of the immune sera, the Newcastle B1 strain were blotted onto PVDF and incubated with antisera from rHN and rF immunized mice.
3.6. ELISA Analysis of the Recombinant Proteins
The sera from the immunized mice were assayed for the presence of specific antibody by ELISA test. The purified rHN and rF (500 ng per well) in 100 μL bicarbonate buffer (15 mM Na2CO3 and 35 mM NaHCO3) were used to coat Maxisorb plates (Nunc, Denmark) overnight at 4 °C. The wells were blocked for additional two hours at 37 °C by covering the well with 200 μL of 5% (w/v) non-fat skim milk in PBST (PBS containing 0.05% Tween-20). After washing with PBST (3 times), the wells were exposed to the diluted immunized serum in a triplicate manner at 100 μL per well for 1 hour at 37 °C. The bound antibodies were detected with the goat anti-mouse IgG conjugated with HRP (Sigma, Germany) in a 1:5000 dilution for 1 hour and washed with PBST (3 times). The reaction was developed with O-phenylenediamine (OPD) as a substrate for HRP (Sigma, Germany) for 15 min at ambient temperature and in a dark place. Sulfuric acid (2.5 M) was added to the reaction and the absorbance in each well was measured at 492 nm.
3.7. Statistical Assessment
SPSS 16.0 was used for all statistical analyses. The statistical significance was considered for the p-value less than 0.05 (p < 0.05). The mean differences were studied by Duncan’s multiple range test.
4. Results
4.1. Construction of the Expression Plasmids
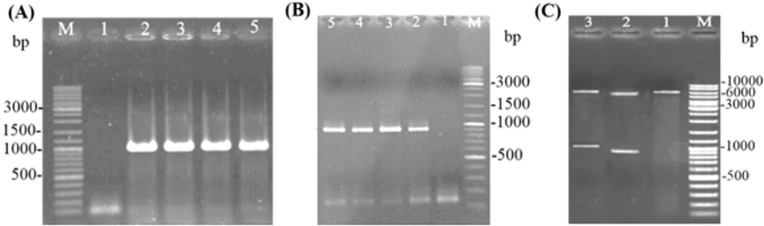
The specific primers were designed to amplify hn and f fragments from the pUC57-hnf plasmid as a template. The PCR product of hn (~1000 bp) and f (~850 bp) was digested (BamHI / XhoI) and cloned in pET32a which was digested with the same enzymes. The proper cloning procedure was confirmed by colony PCR and restriction enzyme digestion (Fig. 1A, Fig. 1B and Fig. 1C). The identity of the hn and f genes in the recombinant plasmids construct was confirmed by the sequencing (data not shown).
Figure 1.
Confirmation of (A) hn (1000 bp) and (B) f (852 bp) genes cloning by PCR and digestion. In (A) and (B), lane 1 corresponds to the non-recombinant plasmid and lane 2 the positive control. (C) The recombinant plasmids were digested with BamHI /XhoI restriction enzymes, lane 1: pET32a (+) (5900 bp), lane 2: pET-32a (+)/f(852 bp), lane 3: pET-32a (+)/hn (1000 bp), and M: DNA Ladder Mix (Thermo).
4.2. The Expression and Purification of the Recombinant Proteins
The expression of recombinant proteins was analyzed on 12% SDS-PAGE and the two fragment: rHN (54 kDa) and rF (49 kDa) in the fusion with Trx-tag and 6x His-tag (N-terminal) were detected (Fig. 2A and Fig. 2B). The expressed recombinant proteins produced as inclusion bodies (IB) (Fig. 2C and Fig. 2D) were subsequently solubilized using 8 M (Fig. 3A). The rHN and rF were produced as approximately 40% of the total bacterial proteins and the highest detectable level of the purified rHN and rF were calculated up to 0.37 and 0.45 mg.mL-1, respectively.
Figure 2.
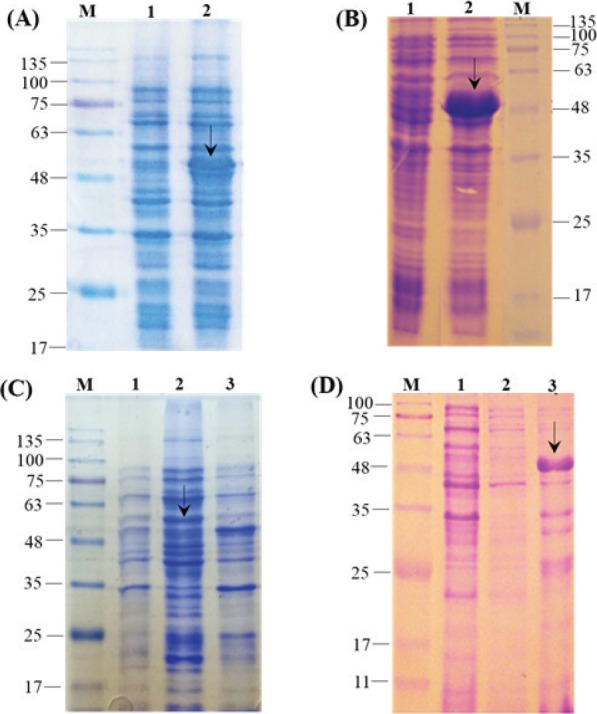
(A) SDS-PAGE analysis of the total bacterial proteins containing pET32a-hn (lane 2, ~54 kDa), (B) pET32a-f (lane 2, ~49 kDa); in both gels lane 1 corresponds to before induction condition. (C) Determination of target proteins’ solubility for rHN (lane 2: insoluble, lane 3: soluble fractions) and (D) rF (lane 2: soluble, lane 3: insoluble fractions), and lane 1 is the non-induced control. M: protein molecular weight marker (KD).
Figure 3.
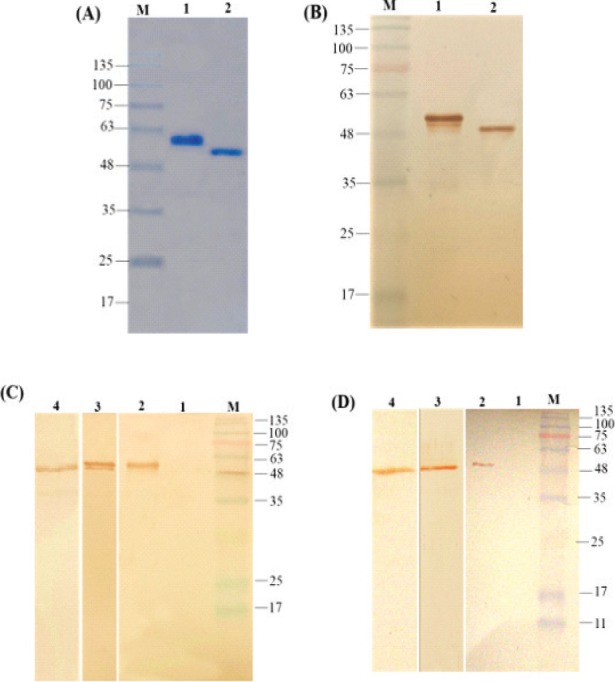
(A) SDS-PAGE analysis of the purified rHN (lane 1) and rF (lane 2) under denaturing conditions. (B) Western blotting of the purified recombinant rHN (lane 1) and rF (lane 2) with anti-His tag antibody. (C) and (D) Western blot analysis of the rHN and rF with specific anti-HN, anti-F (lane 2), and anti-Newcastle B1 (lane 3) antibodies, respectively; lane 4 corresponds to the cross-reactivity analyses of the Newcastle B1 virus strain detected by specific anti-HN and anti-F antibodies, respectively. Lane 1 in (C) and (D) are the negative control non-recombinant pET32a. M: protein molecular weight marker (KD).
4.3. Immunoblotting of the Recombinant Proteins
The authenticity of the recombinant HN (54 kDa) and F (49 kDa) proteins were confirmed by using anti-poly His-tag antibody (Fig. 3B). Moreover, the presence of HN and F recombinant proteins were recognized by the specific anti-HN, anti-F, and anti-Newcastle B1 antibodies (Fig. 3C and Fig. 3D; lane 2). The anti-Newcastle B1 antibody which was produced in the immunized mice was used to characterize the purified rHN and rF proteins (Fig. 3C and Fig. 3D; lane 3). It was suggested that the two epitopic proteins possess antigenicity and the specific reactivity with the Newcastle B1 strain. The bands of approximate 49 kDa and 54 kDa (Fig. 3C and Fig. 3D; lane 4) clearly showed the robust and specific immune reaction between the selected antigens and a standard B1 virus strain. In contrast, there was no reactivity in the negative control (Fig. 3C and Fig. 3D; lane 1).
4.4. ELISA and Cross-Reactivity Analysis
The ELISA assay was used to determine the quantity of the specific IgG antibodies against HN and F purified proteins. The specific IgG antibodies against HN and F fragments were detected after the first immunization in the sera of the immunized mice and were increased significantly after the second booster (Fig. 4A and Fig. 4B). There was a significant difference (p < 0.05) in the antibody titers between rHN and rF in the first injection. As previously mentioned, Western blot data indicated that the serum of the immunized animal with the Newcastle B1 strain could detect rHN and rF proteins and vice versa. The ELISA results confirmed these findings and showed that the higher dilutions of the anti-Newcastle B1 antibody reacted with the very low amount (i.e., 100 ng) of the purified rHN and rF. Despite some differences in endpoint titers of the antibodies, they were not statistically significant (p < 0.05, Fig. 4C).
Figure 4.
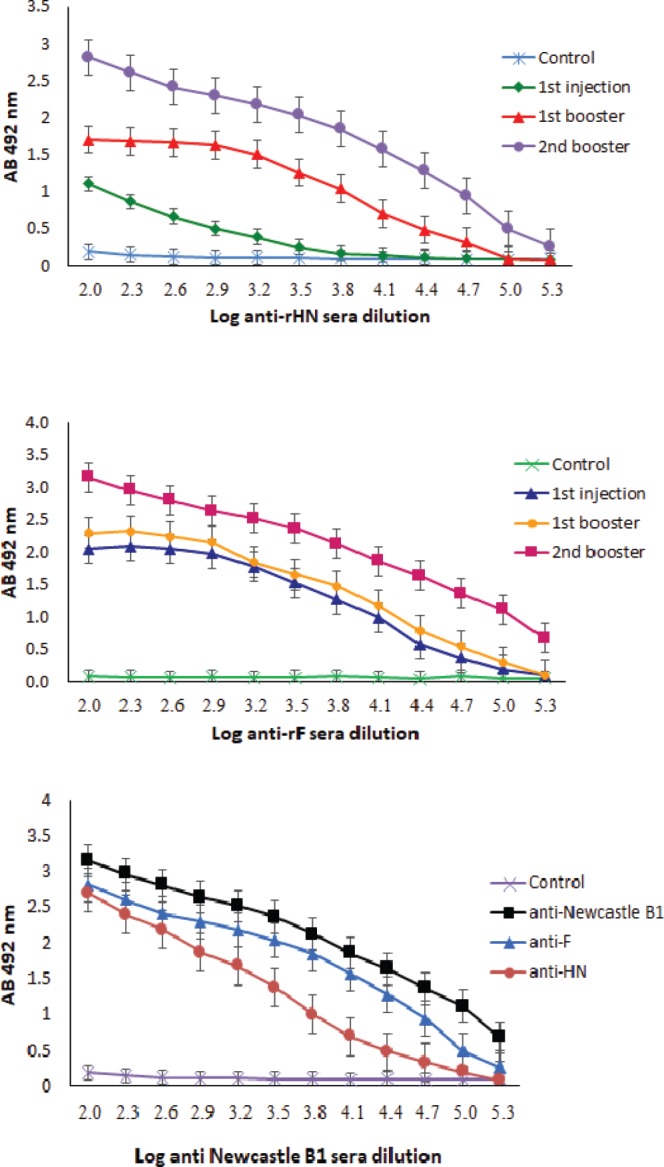
The IgG response to rHN (A) and rF (B) in the immunized mice sera. (C) Titration of the sera from Newcastle B1 mice immunized with rHN, rF, and Newcastle B1.
To evaluate the cross-reactivity of the recombinant proteins (rHN and rF), the sera from mice immunized with the Newcastle B1 were collected at 14 and 28 days post first immunization (dpi), and ELISA assay was conducted to determine the specific antibody titers (Fig. 5A and Fig. 5B). Titration of the anti-HN and anti-F antibodies was monitored and compared with the anti-Newcastle virus B1 strain. The titer of the anti-Newcastle B1, anti-HN, and anti-F reached at the peak level after 28 dpi and temporal variation between all-time points were significant (p < 0.05). Sera from the mice immunized with the Newcastle B1 could cross-react with the pure rHN and rF, respectively (Fig. 5A and Fig. 5B). The antibody titers specific to both rHN and rF were not detected in the control group injected with PBS (data not shown).
Figure 5.

Estimation of serum antibody response and cross-reactivity analyses of the antibodies from the immunized mice versus recombinant purified proteins rHN (A) and rF (B) by ELISA at various days post-first injection. The comparison between samples was conducted using the Duncan test at the 5% level, and the error bars are ± 1 SD. Values within a column followed by different letters are significantly different (p < 0.05).
5. Discussion
The production of the viral antigens through recombinant technology has provided us with an opportunity to use these protective regions from the virus structure as safe and non-replicating immunogens. The recombinant ND vaccines harboring the HN and F proteins’ genes have been constructed using various expression systems mainly baculovirus (27), cytomegalovirus (28), and plants systems (29). The protection against the NDV has been demonstrated by using the F, HN, or both proteins produced in the viral vectors as successful vaccines (30). In this study, we have shown that recombinant multi-epitopic regions of HN and F glycoproteins, instead of the whole molecule, can be produced efficiently in the E. coli as host cells. Our findings showed that recombinant proteins of the multi-epitopic HN and F of the NDV could be detected by the sera from the immunized mice with the Newcastle B1 virus strain and vice versa. Despite this finding, a detailed study on the combination of these two antigens for the development of serological tests has become necessary.
In order to overcome the problem of inclusion body production in the expression of the recombinant proteins, HN and F, pET32a vector was used. This vector encodes 109 aa trxA (Trx-tag) domain at the 5’-end of its multiple cloning sites immediately upstream S-Tag (15 aa) sequence that theoretically has to improve the solubilization of the target proteins. However, the fusion of the HN and F polypeptide to the N-terminus of Trx protein did not lead to the expression of rHN and rF in the soluble fraction of the bacterial cell lysate. It seems that the insolubility of the chimeric HN and F could be attributed to the hydrophobic nature of several strong hydrophobic residues in the different regions of the proteins, thereby making them difficult to solubilized, isolate or purify (31). It was proven that the degree of the soluble expression versus that of insoluble protein in the fusion constructs is dependent on the nature of the target protein (32). In fact, recombinant proteins which expressed in the E. coli cytoplasm is partially insoluble due to hydrophobic residues which distributed on the surface of the proteins (33). Other studies have reported the soluble expression of HN or F in E. coli host in which the vectors with a fusion partner such as Trx and NusA have been used (16, 17). It was revealed that the expression of subunits of the heteromultimeric proteins in the soluble form sometimes results in their aggregation as the inclusion body in absence of a proper fusion tag (33). In a similar research, Wong et al. (16) have reported that the insoluble rHN protein elicits a more rapid and stronger immune response in compression to the soluble NusA HN protein.
It was demonstrated that the insoluble protein aggregates in form of inclusion bodies have an advantage in the protein purification process since they can be harvested from the lysed cells using a moderate-speed centrifugation and also be protected against the proteolytic degradation by the host cell proteases (32). Moreover, the concentration of the solubilizing agent slowly decreases during dialysis which allows the protein to refold optimally (34). The minimal aggregation of the purified recombinant HN and F proteins was achieved by the step-wise decrease in the urea concentration (8M to 0 M) in order to ensure a proper solubilization and may also refold of the proteins to their native conformation.
It was found that the HN and F proteins could stimulate neutralizing antibody responses and was considered as effective and protective antigens (35). Studies using monoclonal antibodies have revealed the presence of various epitopes within the HN (36) and F protein (37). Since the goal of this study was to use these proteins in the immunodetection, the existence of the antibody against HN and F antigens in the serum of the mice compared to that of the control group was evaluated by the Western blot to confirm antigenic characteristic of the recombinant proteins. Whereas blotting with the serum of immunized mice has had always background due to general antibodies against E. coli proteins, it is better the results of Western blotting first become subject of comparison before and after induction for the presence or absence of the desired band (Fig. 4C and Fig. 4D). Moreover, the cross-reactivity of the multi-epitopic recombinant protein antigens with serum samples from mice immunized with B1 strain of NDV was analyzed.
Furthermore, Western blotting analysis could only show the reaction of the continuous epitopes and the corresponding antibodies because conformational epitopes denatured in the presence of SDS detergent (38). The immunoblotting results showed an appropriate interaction between all immunized mice sera raised against rHN and rF proteins. In this regard, several studies have reported the nature of the epitopes present on the immune-blotted HN and F proteins in which the continuous epitopes were able to regenerate after denaturation in SDS-PAGE (37, 39-41). Furthermore, E. coli cells lack metabolic pathways for glycosylation and are unable to add oligosaccharide chain to the recombinant proteins (20). Therefore, binding of the antibodies to the recombinant HN and F proteins was limited to the involvement of the linear epitopes devoid of carbohydrates.
Mice administrated with the rF protein showed significant antibody titers two weeks after the first injection. The antibody titers had a constant trend even after the first booster of the rF protein (log = 2.04-2.28). After the second injection, the titers went up steadily to log = 2.84 (Fig. 5B). In contrast, mice injected with the recombinant HN protein did not show an increase in their response by the production of antibody until the booster injection was given. The titer of antibody was increased slightly from log 10 : 1.10-1.70 after the first injection of the rHN protein and ultimately reached a significant level of log 10 : 2.31 (Fig. 5A). The recombinant HN and F proteins in the inclusion bodies were in the state of protein aggregates which could increase the likelihood of the effective T-cell epitope response and engage the antigen-processing cells (42). Analysis of cross-reactive antibodies against the recombinant purified proteins in the mice has indicated that HN and F proteins could elicit the immune response against the both molecules. Results of the experiment showed that the muti-epitopic recombinant HN and F antigens expressed in the E. coli could elicit a sufficient rise of antibody in the immunized mice for detection by ELISA as well as standard Newcastle vaccine which composed of the whole structural NDV proteins. On the other hand, the commercial strains that are currently being used for detection of NDV induce a high amount of antibodies which may not directly produce against HN or F antigens, because NDV structural proteins like nucleocapsid protein (NP) are also associated with the high immunogenicity properties, as well (17, 43).
Despite an equal amount of these recombinant proteins, the antibody responses to F and HN were not equal at the beginning. A comparison of the endpoint titers showed that there are not statistically significant differences. These results were in line with the findings of other researchers using similar epitopic or the full-length of F or HN protein for immunogenicity in the animal models (16, 17, 19). Furthermore, the detection of the HN and F glycoproteins with the individually produced antibodies revealed the potential application of these antibodies as probes for the viral glycoprotein in immunological analyses. The reactivity of the anti-NDV serum toward the denatured recombinant HN and F proteins in ELISA opens the possible use of these recombinant proteins as antigens for diagnosis or vaccine survey of ND in poultry.
6. Conclusions
There are limited reports about the successful expression of NDV glycoproteins in E.coli as the host. In this investigation, using bioinformatics tools we miniaturized the structure of the HN and F with the appropriate design while keeping the immune properties of the recombinant glycoproteins under the defined conditions. Moreover, our finding has provided an outstanding information about the cross-reactivity properties in addition to the biological activity of the synthetic recombinant proteins in comparison to the standard B1 strain of the NDV. These findings suggest the proper presentation of the antigens from recombinant glycoproteins to the animal immune system, which raises specific antibodies with the high affinity toward native proteins in NDV structure. Further investigations are needed to obtain better antibody titers in the mice serum or to generate potential immunogenicity in a chicken model which could be applicable as a practical approach for detection of NDV.
Acknowledgements
The National Institute of Genetic Engineering and Biotechnology financially supported this work (NIGEB grant number: 447).
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Miller PJ, Decanini EL, Afonso CL. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect Genet Evol. 2010;10(1):26-35. doi: 10.1016/j.meegid.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 2.Alexander DJ, Aldous EW, Fuller CM. The long view: a selective review of 40 years of Newcastle disease research. Avian Pathol. 2012;41(4):329-335. doi: 10.1080/03079457.2012.697991 [DOI] [PubMed] [Google Scholar]
- 3.Lim MAG. Newcastle Disease Vaccines. Commercial Plant-Produced Recombinant Protein Products. 68. Berlin Heidelberg: Springer; 2014. p. 179-195. [Google Scholar]
- 4.Lamb RA, Jardetzky TS. Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol. 2007;17(4):427-436. doi: 10.1016/j.sbi.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Gorman JJ, McKimm-Breschkin J, Lawrence LJ, Tulloch PA, Smith BJ, Colman PM, Lawrence MC. The structure of the fusion glycoprotein of Newcastle disease virus suggests a novel paradigm for the molecular mechanism of membrane fusion. Structure. 2001;9(3):255-266. doi: 10.1006/viro.2001.1172 [DOI] [PubMed] [Google Scholar]
- 6.Takimoto T, Taylor GL, Connaris HC, Crennell SJ, Portner A. Role of the hemagglutinin-neuraminidase protein in the mechanism of paramyxovirus-cell membrane fusion. J Virol. 2002;76(24):13028-13033. doi: 10.1128/JVI.76.24.13028-13033.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly SA, Leser GP, Jardetzky TS, Lamb RA. Bimolecular complementation of paramyxovirus fusion and hemagglutinin-neuraminidase proteins enhances fusion: implications for the mechanism of fusion triggering. J Virol. 2009;83(21):10857-10868. doi: 10.1128/JVI.01191-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melanson VR, Iorio RM. Amino acid substitutions in the F-specific domain in the stalk of the Newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J Virol. 2004;78(23):13053-13061. doi: 10.1128/JVI.78.23.13053-13061.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyoda T, Gotoh B, Sakaguchi T, Kida H, Nagai Y. Identification of amino acids relevant to three antigenic determinants on the fusion protein of Newcastle disease virus that are involved in fusion inhibition and neutralization. J Virol. 1988;62(11):4427-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iorio RM, Glickman RL, Sheehan JP. Inhibition of fusion by neutralizing monoclonal antibodies to the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J Gen Virol. 1992;73(5):1167-1176. doi: 10.1099/0022-1317-73-5-1167 [DOI] [PubMed] [Google Scholar]
- 11.Hooper DC. Plant vaccines: an immunological perspective. Plant-produced Microbial Vaccines: Springer; 2009. p. 1-11. doi: 10.1007/978-3-540-70868-1_1 [DOI] [PubMed] [Google Scholar]
- 12.Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm. 2008;364(2):272-80. doi: 10.1016/j.ijpharm.2008.04.036 [DOI] [PubMed] [Google Scholar]
- 13.De Groot AS, Berzofsky JA. From genome to vaccine--new immunoinformatics tools for vaccine design. Methods. 2004;34(4):425-428. doi: 10.1016/j.ymeth.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1(3):209-219. doi: 10.1038/35105075 [DOI] [PubMed] [Google Scholar]
- 15.World Organization for Animal Health (OIE). Newcastle disease: Manual of dignosis tests and vaccines for tresstrial animals. 6th ed. Paris, France 2012. p. 1-19. [Google Scholar]
- 16.Wong SK, Tan WS, Omar AR, Tan CS, Yusoff K. Immunogenic properties of recombinant ectodomain of Newcastle disease virus hemagglutinin-neuraminidase protein expressed in Escherichia coli. Acta Virol. 2009;53(1):35-41. doi: 10:4149/av_2009_01_35 [DOI] [PubMed] [Google Scholar]
- 17.Moon HJ, Park JE, Yoon H, Cruz DJM, Kim CJ, Shin HJ. Development of a novel recombinant heamagglutinin-neuramindase Elisa (rHN-ELISA) for evaluation of humoral immunity in chicken vaccinated against Newcastle disease virus (NDV). J. Anim. Vet. Adv. 2010;9(23):2932-2939. doi: 10.3923/javaa.2010.2932.2939 [Google Scholar]
- 18.Frozandeh-Moghadam M, Madani R, Dehghani M, Mosavi S, Pourbakhsh S, Golchinfar F. Cloning and expression of F protein gene (HR1 region) of Newcastle disease virus NR43 isolate from Iran in E. coli. Iran J Virol. 2009;3(3):16-22. [Google Scholar]
- 19.Shahid N, Tahir S, Rao AQ, Hassan S, Khan A, Latif A, Khan MA, Tabassum B, Shahid AA, Zafar AU. Escherichia coli expression of NDV fusion protein gene and determination of its antigenic epitopes. Biologia (Bratisl). 2015;70(12):1553-1564. doi: 10.1515/biolog-2015-0191 [Google Scholar]
- 20.Schmidt F. Recombinant expression systems in the pharmaceutical industry. Appl Microbiol Biotechnol. 2004;65(4):363-372. doi: 10.1007/s00253-004-1656-9 [DOI] [PubMed] [Google Scholar]
- 21.Sadeghi M, Bandehpour M, Yarian F, Yassaee V, Torbati E, Kazemi B. Cloning and expression of influenza H1N1 NS1 protein in Escherichia Coli BL21. Iran J Biotechnol. 2014;12(1):25-29. doi: 10.5812/ijb.12625 [Google Scholar]
- 22.Amorim JH, Porchia BF, Balan A, Cavalcante RC, da Costa SM, de Barcelos Alves AM, de Souza Ferreira LC. Refolded dengue virus type 2 NS1 protein expressed in Escherichia coli preserves structural and immunological properties of the native protein. J Virol Methods. 2010;167(2):186-192. doi: 10.1016/j.jviromet.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Liu K, Jiang Y, Wei J-C, Chen P-Y. Multiple linear B-cell epitopes of classical swine fever virus glycoprotein E2 expressed in E. coli as multiple epitope vaccine induces a protective immune response. Virol J. 2011;8(1):1-7. doi: 10.1186/1743-422X-8-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talha SM, Nemani SK, Salminen T, Kumar S, Swaminathan S, Soukka T, Pettersson K, Khanna N. Escherichia coli-expressed near full length HIV-1 envelope glycoprotein is a highly sensitive and specific diagnostic antigen. BMC Infect Dis. 2012;12(1):1-11. doi: 10.1186/1471-2334-12-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Zaro JL, Shen W-C. Fusion protein linkers: property, design and functionality. Adv Drug Delivery Rev. 2013;65(10):1357-1369. doi: 10.1016/j.addr.2012.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motamedi MJ, Amani J, Shahsavandi S, Salmanian AH. In Silico design of multimeric HN-F antigen as a highly immunogenic peptide vaccine against Newcastle disease virus. Int J Peptide Res Therapeut. 2014;20(2):179-194. doi: 10.1007/s10989-013-9380-x [Google Scholar]
- 27.Zoth SC, Gómez E, Carballeda JM, Carrillo E, Berinstein A. Expression of a secreted version of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus Its evaluation as a diagnostic reagent. J Vet Diagn Invest. 2011;23(3):519-523. doi: 10.1177/1040638711404153 [DOI] [PubMed] [Google Scholar]
- 28.Loke C, Omar AR, Raha A, Yusoff K. Improved protection from velogenic Newcastle disease virus challenge following multiple immunizations with plasmid DNA encoding for F and HN genes. Vet Immunol Immunopathol. 2005;106(3):259-267. doi: 10.1016/j.vetimm.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 29.Lai KS, Yusoff K, Mahmood M. Functional ectodomain of the hemagglutinin-neuraminidase protein is expressed in transgenic tobacco cells as a candidate vaccine against Newcastle disease virus. Plant Cell Tiss Org. 2013;112(1):117-121. doi: 10.1007/s11240-012-0214-x [Google Scholar]
- 30.Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. Nonsegmented negative-strand viruses as vaccine vectors. J Virol. 2006;80(21):10293-10306. doi: 10.1128/JVI.00919-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yusoff K, Tan WS. Newcastle disease virus: macromolecules and opportunities. Avian Pathol. 2001;30(5):439-455. doi: 10.1080/03079450120078626 [DOI] [PubMed] [Google Scholar]
- 32.Hwang PM, Pan JS, Sykes BD. Targeted expression, purification, and cleavage of fusion proteins from inclusion bodies in Escherichia coli. FEBS Lett. 2014;588(2):247-252. doi: 10.1016/j.febslet.2013.09.028 [DOI] [PubMed] [Google Scholar]
- 33.Sørensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact. 2005;4(1):1-8. doi: 10.1186/1475-2859-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi H, Miyazaki M. Refolding techniques for recovering biologically active recombinant proteins from inclusion bodies. Biomolecules. 2014;4(1):235-251. doi: 10.3390/biom4010235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun HL, Wang YF, Tong GZ, Zhang PJ, Miao DY, Zhi HD, Wang M, Wang M. Protection of chickens from Newcastle disease and infectious laryngotracheitis with a recombinant fowlpox virus co-expressing the F, HN genes of Newcastle disease virus and gB gene of infectious laryngotrachei tis virus. Avian Dis. 2008;52(1):111-117. doi: 10.1637/7998-041807-Reg [DOI] [PubMed] [Google Scholar]
- 36.Iorio RM, Glickman RL, Riel AM, Sheehan JP, Bratt MA. Functional and neutralization profile of seven overlapping antigenic sites on the HN glycoprotein of Newcastle disease virus: monoclonal antibodies to some sites prevent viral attachment. Virus Res. 1989;13(3):245-261. doi: 10.1016/0168-1702(89)90019-1 [DOI] [PubMed] [Google Scholar]
- 37.Yusoff K, Nesbit M, McCartney H, Meulemans G, Alexander D, Collins M, Emmerson PT, Samson ACR. Location of neutralizing epitopes on the fusion protein of Newcastle disease virus strain Beaudette C. J Gen Virol. 1989;70(11):3105-3109. doi: 10.1099/0022-1317-70-11-3105 [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y-H, Chen Z, Purcell RH, Emerson SU. Positive reactions on Western blots do not necessarily indicate the epitopes on antigens are continuous. Immunol Cell Biol. 2007;85(1):73-78. doi: 10.1038/sj.icb.7100004 [DOI] [PubMed] [Google Scholar]
- 39.Long L, Portetelle D, Ghysdael J, Gonze M, Burny A, Meulemans G. Monoclonal antibodies to hemagglutinin-neuraminidase and fusion glycoproteins of Newcastle disease virus: relationship between glycosylation and reactivity. J Virol. 1986;57(3):1198-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers P, Nesbit M, Yusoff K, Millar N, Samson A, Emmerson P. Location of a neutralizing epitope for the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J Gen Virol. 1988;69(8):2115-2122. [DOI] [PubMed] [Google Scholar]
- 41.Panshin A, Shihmanter E, Weisman Y, Örvell C, Lipkind M. Variability of antigenic epitopes of the fusion protein of Newcastle disease virus. Comp Immunol Microbiol Infect Dis. 1998;21(1):51-63. doi: 10.1016/S0147-9571(97)00016-7 [DOI] [PubMed] [Google Scholar]
- 42.Yin L, Chen X, Tiwari A, Vicini P, Hickling TP. The role of aggregates of therapeutic protein products in immunogenicity: an evaluation by mathematical modeling. J Immunol Res. 2015;2015: 1-14 doi: 10.1155/2015/401956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva KR, Goncalves MCM, de Oliveira ES, Fernando FS, Montassier MdFS, Fernandes CC, Tamanine MdLF, Borzi MM, dos Santos RM, de Oliveira Mendonca A. Cloning and expression of the nucleoprotein gene (NP) of Newcastle disease virus (NDV) in Escherichia coli for immunodiagnosis application. Int J Poultry Sci. 2014;13(8):473-479. doi: 10.3923/ijps.2014.473.479 [Google Scholar]