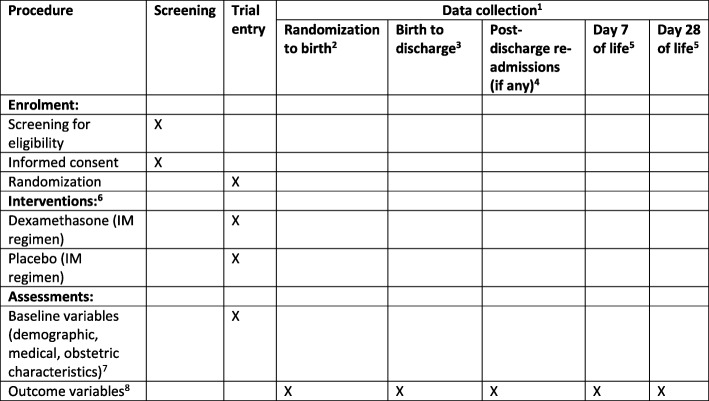
Fig. 1.
SPIRIT figure for the Antenatal Corticosteroids for Improving Outcomes in preterm Newborns (ACTION)-I trial. 1 Data will be collected from randomized women from time of randomization to day 28 postpartum. Data will be collected on all newborn babies (single or multiple) from time of birth to day 28 postnatal or death. 2 Data will be collected from time of randomization. Some randomized women may be discharged without giving birth; however, data collection continues when they are readmitted later in the pregnancy for birth. 3 Data will be collected for women and newborn babies during admission until discharge. If the length of admission exceeds 28 days, data will be collected to 28 completed days only. 4 Women or newborn babies who experience a readmission to hospital during the follow-up period (postdischarge from hospital following birth) will have data collected. 5 Day 7 and day 28 follow-up visits will be performed, regardless of location (hospital or community). 6 The regimen is described in the study protocol. A full course (four doses) takes a total of 36 h to administer. In the event that a randomized woman does not give birth within 7 days, she may be eligible for a repeat course (four doses). 7 Baseline variables are collected after the first dose has been administered. Baseline variables include: age, education, marital status, gravidity, parity, maternal history of preterm birth, weight, height, mid-upper arm circumference, medical conditions (chronic hypertension, diabetes mellitus, HIV/AIDS, tuberculosis, pyelonephritis, anaemia, malaria), obstetric conditions (gestational diabetes, preterm prelabour rupture of membranes, pre-eclampsia or eclampsia, gestational hypertension, oligohydramnios, polyhydramnios, intrauterine growth restriction (known or suspected), macrosomia, abruptio placentae, placenta praevia, other obstetric haemorrhage), gestational, use of tocolysis, symptoms of imminent preterm birth. 8 All outcome variables are described in Additional file 2. IM intramuscular