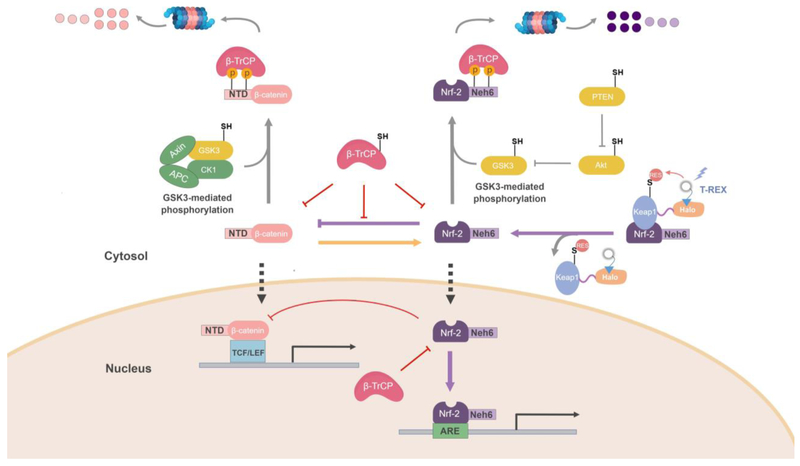
Figure 4. Study of AR signaling under Keap1-specific RES-modification reveals an unexpected intersection between β-catenin/Wnt and Keap1/Nrf-2 axes, in which β-TrCP sensitizes Wnt signaling to AR-mediated Wntpathway-inhibition[52].
Nrf-2 strongly inhibits β-catenin/Wnt signaling, whereas β-catenin overexpression upregulates AR. Both Nrf-2 and β-catenin are subject to proteasomal degradation mediated by β-TrCP. β-TrCP’s binding occupancy at the N-terminal domain (NTD) of β-catenin protects β-catenin against Nrf2-mediated inhibition. To prevent β-TrCP-promoted degradation of β-catenin, the β-catenin-NTD is frequently mutated in cancers: these NTD-mutations upregulate Wnt-signaling. However, impeding β-TrCP-binding to β-catenin renders β-catenin signaling more susceptible to Nrf2/AR-mediated inhibition. This novel regulatory mechanism was uncovered only as a result of studying RES-induced AR-upregulation in a Keap1-specific manner. This delicate regulation and crosstalk between β-catenin/Wnt and Keapt1/Nrf-2-signaling pathways are masked during whole-cell RES-stimulation. Known/postulated redox-sensitive players are labeled with “–SH”.