Abstract
Object
Low back pain (LBP) attributable to fusion failure, implant failure, infection, malalignment, or adjacent segment disease may persist after lumbar fusion surgery (LFS). Superior cluneal nerve (SCN) entrapment neuropathy (SCNEN) is a clinical entity that can produce LBP. We report that SCNEN treatment improved LBP in patients who had undergone LFS.
Methods
Between April 2012 and August 2015, we treated 8 patients (4 men and 4 women ranging in age from 38 to 88 years; mean age, 69 years) with SCNEN for their LBP after LFS. Our criteria for the diagnosis of SCNEN included a trigger point over the posterior iliac crest 7 cm from the midline and numbness and radiating pain in the SCN area upon compression of the trigger point. Symptom relief was obtained in more than 75% of patients within 2 h of inducing a local nerve block at the trigger point in the buttocks. The mean postoperative follow-up period was 28 months (range, 9-54 months).
Results
LBP was unilateral in 3 and bilateral in 5 patients. The senior author (T.I.) operated all patients for SCNEN under local anesthesia because they reported recurrence of pain after the analgesic effect of repeat injections wore off. This led to a significant improvement of their LBP.
Conclusions
SCNEN should be considered in patients reporting LBP after LFS. Treatment of SCNEN may be a useful option in patients with failed back surgery syndrome after LFS.
Keywords: Superior cluneal nerve entrapment neuropathy, low back pain, lumbar fusion surgery
Introduction
While lumbar fusion surgery (LFS) is an accepted surgical procedure, 5%-30% of patients report postoperative low back pain (LBP) known as failed back surgery syndrome (FBSS)1-3). Superior cluneal nerve (SCN) entrapment neuropathy (SCNEN) involving the iliac crest can prompt LBP whose features and etiology are poorly understood. As LBP due to SCNEN is aggravated by lumbar movement, it may be misdiagnosed as lumbar spine disease4,5).
We report that the treatment of SCNEN improved LBP in patients who had undergone LFS.
Methods
Criteria for the Diagnosis of SCNEN
We elsewhere reported our diagnostic criteria for SCNEN, i.e., unilateral LBP involving the iliac crest and buttocks, a trigger point over the posterior iliac crest located approximately 7 cm lateral from the midline (corresponding to the nerve entrapment point), and numbness and radiating pain in the SCN area upon compression of the trigger point4,5). For diagnostic purposes, we blocked the SCN by injecting 2 mL of 1% lidocaine at the trigger points in the buttock; the drug had no effect on pain from other causes, such as sacroiliac joint pain4,5). Symptom relief of more than 75% was obtained within 2 h of inducing the nerve block, and a 75% pain reduction confirmed the diagnosis4,5).
Patient Population
Between April 2012 and August 2015, we admitted 30 patients who had undergone LFS and were dissatisfied with outpatient observation of their LBP. We excluded 12 patients from this study who had acute fracture and malignancy, infection, or other bone diseases other than osteoporosis; patients whose LBP was controlled by acetaminophen and/or non-steroidal anti-inflammatory drugs (NSAIDs); patients with iliac crest harvest for grafting; patients with dementia or psychological disorders; and patients complaining of a radiating leg pain. Of the other 18 patients, 8 were diagnosed with SCNEN based on published diagnostic criteria4,5) and were enrolled in this study.
The included patients comprised 4 men and 4 women ranging in age from 38 to 88 years (mean age, 69 years). The affected side was unilateral in 3 patients and bilateral in 5. The duration from onset to treatment averaged 29.3 months (range, 12-96 months). We carefully assessed the patients to ascertain that their LBP was attributable to SCN entrapment. The mean follow-up period after SCNEN treatment was 28 months (range, 9-54 months). Prior written informed consent for participation in this investigation was obtained from all patients (Table 1).
Table 1.
Clinical Profiles of 8 Patients with SCNEN after Lumbar Fusion Surgery.
| Case | Gender | Age (years) |
Fusion level |
Interval after LFS (months) |
Interval re-LBP after (months) LFS (months) |
Persistence or Recurrence of LBP |
SCNEN surgery |
NRS | RDQ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Tr |
After Tr |
Before Tr |
After Tr |
||||||||
| 1 | F | 62 | L5/S | 338 | 264 | Recurrence | Bil | 8 | 0 | 15 | 3 |
| 2 | F | 74 | L4/5 | 60 | 48 | Recurrence | Bil | 9 | 0 | 12 | 0 |
| 3 | M | 72 | L2-S1 | 24 | 6 | Recurrence | Bil | 9 | 1 | 20 | 6 |
| 4 | M | 70 | L4/5 | 96 | 84 | Recurrence | Bil | 8 | 0 | 10 | 0 |
| 5 | M | 68 | L3-5 | 24 | 18 | Recurrence | Bil | 7 | 0 | 8 | 0 |
| 6 | F | 88 | L5/S1 | 98 | 84 | Recurrence | Rt | 10 | 0 | 14 | 7 |
| 7 | F | 77 | L3-5 | 60 | 24 | Recurrence | Lt | 8 | 2 | 8 | 4 |
| 8 | M | 38 | L3/4 | 12 | 0 | Persistent | Lt | 8 | 0 | 10 | 1 |
F: female, M: male, NRS: numerical rating scale, L: lumbar, S: sacrum, Lt: left, Rt: right, Bil: bilateral, LFS: lumbar fusion surgery, LBP: low-back pain, Interval after LFS: time between LFS and first visit to our hospital, Interval re-LBP after LFS: time between LFS and LBP recurrence, SCNEN: superior cluneal nerve entrapment neuropathy, Tr: SCNEN treatment, RDQ: Roland-Morris Disability Questionnaire, NRS: numerical rating scale
Radiological Findings
Before SCNEN treatment, we obtained 3 lateral lumbar spine radiographs (flexed, neutral, and extended). Neutral-position films without instructions given to the patients were acquired to establish their natural posture. The angle of lumbar lordosis was measured from the superior endplate of L-1 to the superior endplate of S-16,7). The sacral slope is the angle between the superior endplate of S-1 and a horizontal line7,8). The criteria for instability were slippage by more than 4 mm and/or an angle change of more than 10° on flexion and extension9,10). We were unable to evaluate imaging findings obtained before LFS because none of the 8 patients underwent the procedure at our hospital.
Fusion failure based on intervertebral mobility was evaluated on lateral radiographs obtained in flexion and extension. In patients whose intervertebral mobility range was 2° or greater, fusion failure was recorded11-13). Degeneration, such as disc degeneration, spondylolisthesis, instability, herniated nucleus pulposus, spinal canal stenosis, hypertrophic facet arthritis, osteophyte formation, scoliosis, and vertebral compression fractures that develop at segments directly above or below a fused spinal segment, is known as adjacent segment disease (ASD)10). Imaging findings were evaluated by 2 spinal surgeons (M.I. and J.M.) who did not operate on these patients. They performed 3 measurements and used their mean value to diagnose instability, fusion failure, and ASD.
Surgical Procedure
As we reported elsewhere4,5), microsurgical release of the SCN entrapment was performed with the patient in the prone position and under local anesthesia. A 5-cm-long skin incision was made across the trigger point located 7 cm from the midline on the iliac crest. Then, the subcutaneous soft tissue was carefully dissected, and SCN was identified using a nerve stimulator placed on the fat layer over the subcutaneous space. SCN lies obliquely from rostromedial to caudolateral and penetrates the thoracolumbar fascia through the orifice just before crossing over the iliac crest. We then cut the thoracolumbar fascia until we reached a point where SCN was free of kinks and opened the orifice in the distal to rostral direction along SCN. In patients where decompression by SCN neurolysis proved difficult, we cut SCN.
Immediately after surgery, there were no restrictions imposed by external fixation, and the patients were able to walk freely. The next day they were discharged to pursue their activities of daily living.
SCNEN Treatment Outcomes
The SCNEN treatment outcomes were analyzed by comparing the numerical rating scale (NRS), where 0=no pain and 10=worst pain, for LBP and the Roland-Morris Disability Questionnaire (RDQ) score, a self-administered measure of disability due to LBP, recorded before SCNEN treatment and at the last follow-up. For statistical analysis, we subjected our data to the paired t-test using Statmate III software (ATMS Co. Ltd.). Differences of p<0.05 were considered to indicate statistical significance.
Results
LBP Features
LBP was unilateral in 3 patients and bilateral in 5; all patients suffered LBP before LFS. In one patient, it persisted and in the other 7, it improved after LFS but re-appeared 6-264 months (mean, 75 months) later. Characteristically, LBP worsened with body movements, and the patients reported difficulty with prolonged standing and walking.
Radiological Findings
On radiographs obtained before SCNEN treatment, the patients manifested 31.1° lumbar lordosis (range, 5.9°-60.1°); the sacral slope was 25.8° (range, 14.3°-35.2°). None of the patients presented with fusion failure and infection. ASD was noted in 3 patients; 3 had old vertebral fractures.
Nerve-block Treatment of LBP due to SCNEN
In 8 patients with intractable LBP due to SCNEN, we first performed SCN blocking. LBP was reduced transiently in all patients. SCN block performed an average of 5 times (range, 4-8 times) in the 8 patients failed to yield permanent symptom abatement. Because its effectiveness was transient, 3 patients with unilateral and 5 with bilateral SCNEN underwent SCN neurolysis under local anesthesia.
Surgical Treatment of LBP due to SCNEN
Three patients with unilateral SCNEN underwent ipsilateral neurolysis; in 5 patients with bilateral SCNEN, we performed bilateral neurolysis of SCN. All 8 patients reported LBP abatement after surgery and at the latest follow-up.
There were no surgical complications. LBP was significantly improved at the time of the last follow-up (mean, 28 months; range, 9-54 months). Among the 8 patients, 6 reported LBP improvement and the other 2 continued to experience mild LBP (NRS=1-2/10). At the last follow-up, our patients' NRS improved from 8.7 (range, 7-10) to 0.4 (range, 0-2), and their RDQ scores fell from 12 (range, 8-20) to 1.8 (range, 0-6) (p<0.05) (Fig. 1)
Figure 1.
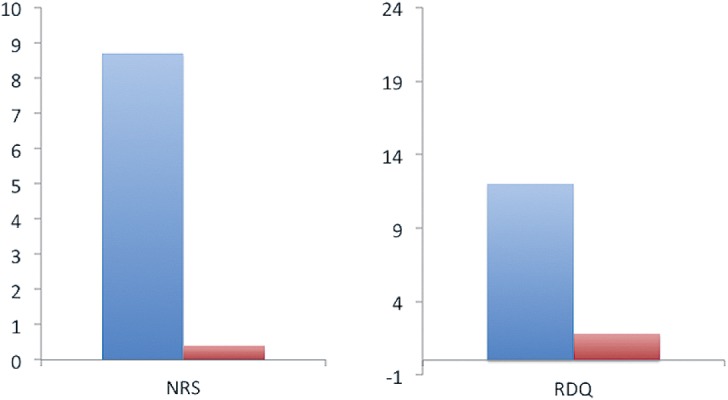
Clinical scores of 8 patients treated by SCNEN surgery. Their scores rose between the first and final follow-up examinations. Their mean NRS for LBP fell from 8.7 (range, 7-10) to 0.4 (range, 0-2), and their mean RDQ scores fell from 12 (range, 8-20) to 1.8 (range, 0-6). NRS: Numeric rating scale, RDQ: Roland-Morris disability questionnaire
Illustrative Case (case 8)
This 38-year-old male patient with LBP had undergone discectomy and LFS 1 year earlier. However, as his LBP persisted despite rest and administration of NSAIDs, he was admitted to our hospital.
At the time of admission, he suffered from LBP in the left iliac crest region, with radiation to the ipsilateral buttock that interfered with his daily activities. It worsened with body movement and gait. Lumbar computed tomography and magnetic resonance imaging findings revealed no causative abnormalities (Fig. 2). His lumbar lordosis and sacral slope were 29.1° and 21.4°, respectively. There was no fusion failure as per our criteria (Fig. 3). We attributed his LBP to left SCNEN and performed SCN blocking. This produced dramatic pain alleviation. However, while his LBP responded to SCN blocks delivered on an outpatient basis, their effects were transient, and his pain returned after a few days. Therefore, he underwent neurolysis for left SCNEN. Postoperatively, his LBP was reduced (NRS: 8-0, RDQ score: 10-1), and he suffered no recurrence during the follow-up period.
Figure 2.
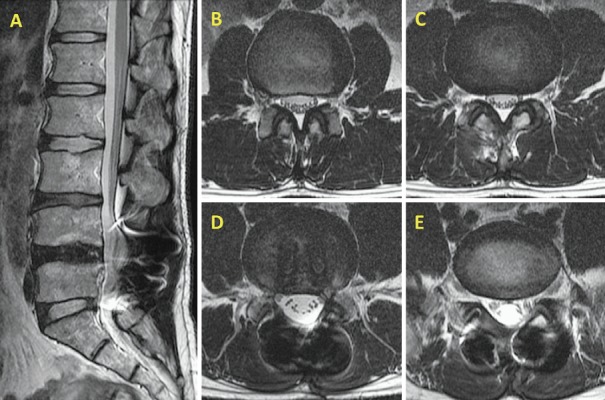
Lumbar MRI (T2WI). Lumbar radiological findings revealed no causative abnormalities.
A. Sagittal image.
B. Axial image on the level of L2/3.
C. Axial image on the level of L3/4.
D. Axial image on the level of L4/5.
E. Axial image on the level of L5/S1.
Figure 3.
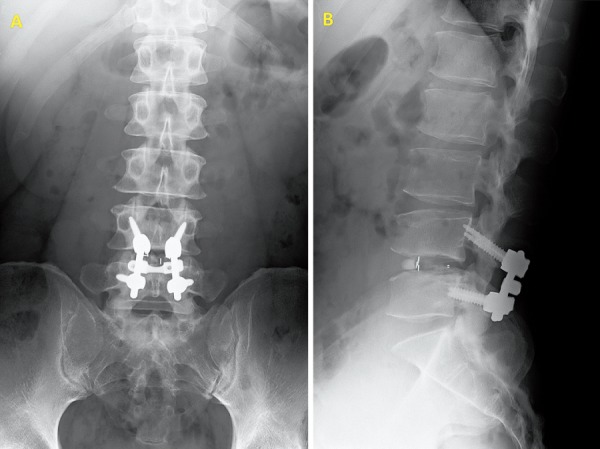
X-ray photograph.
A. Anteroposterior view.
B. Lateral view.
Discussion
LFS used to treat LBP from lumbar disorders yielded better pain relief than conservative treatment14-17). In patients with persistent or new LBP after lumbar surgery, a diagnosis of FBSS is made18-22). The clinical results are not necessarily poor in patients with fusion failure. Post-fusion LBP has been attributed to pseudoarthrosis, infection, malalignment, adjacent level disease, and sacroiliac joint dysfunction1,2,18-22). According to Kuniya et al.23), SCNEN patients experienced no improvement in any symptom after fusion surgery, while SCNEN treatment improved severe LBP after LFS. Likewise, in our 8 patients, SCNEN treatment improved LBP dramatically. Consequently, we suggest that SCNEN is a possible source of persistent or new LBP after LFS and that its treatment may be useful in patients with FBSS after such surgery.
SCN originates at the rami laterales of the posterior branch of the spinal nerve and pierces the thoracolumbar fascia to occupy the cranial buttocks over the iliac crest5,23-25). The SCN branches extend from the site of perforation on the fascia to innervate the buttocks cutaneously5,23-28). Entrapment of SCN at the osteofibrous orifice where it penetrates the thoracolumbar fascia induces LBP26,27,29). In fact, 1.6%-14.0% of LBP has been attributed to SCNEN23,27). Surgical release at the point where SCN penetrates through the osteofibrous orifice is effective5,23,24,27,29). LBP due to SCN entrapment is exacerbated by movements such as rising; sitting; rolling over; crouching; lateral bending and rotating; and by prolonged sitting, standing, or walking5,23,27,28). However, the etiology of SCNEN remains poorly understood. With respect to the relationship between SCNEN and LFS, we documented that postoperative LBP can be addressed by SCNEN treatment.
LBP due to SCNEN occurs as donor-site pain after the harvest of iliac crest bone for grafting30,31). However, none of our patients had undergone iliac bone graft harvesting. Kuniya et al.23) reported that patients with SCNEN experienced no abatement of any symptoms after fusion surgery, suggesting that the operation may have been unnecessary. We suspect that those patients had SCNEN before LFS. In 7 of our 8 patients, LBP improved after LFS but recurred 6-264 (mean, 75) months later. Their clinical course was different from that of the patients reported by Kuniya et al.; their SCNEN may have been missed before LFS and manifested thereafter. We can only offer this hypothesis as our patients had undergone LFS elsewhere before they came to our hospital. Alternatively, LFS may have elicited anatomical changes such as paravertebral muscle atrophy and fatty degeneration that resulted in SCNEN.
Hartwig et al.32) reported paravertebral muscle atrophy and fatty degeneration in some patients who had undergone LFS. Such changes may result in increased traction and entrapment of SCN in the paravertebral muscle and in LBP exacerbation. Biomechanical studies showed that contraction of the paravertebral muscle affects the tension of the thoracolumbar fascia, especially below the level of L433); this may lead to SCNEN. SCN runs in the paravertebral muscle; then, it runs between the muscle and the thoracolumbar fascia and penetrates the thoracolumbar fascia. LFS may result in adhesion of SCN before it penetrates the thoracolumbar fascia, and this may ease traction elicited by lumbar movement.
Persistent or new LBP after LFS has been attributed to pseudoarthrosis, infection, malalignment, adjacent level disease, sacroiliac joint dysfunction, and SCNEN; therefore, re-operation of the lumbar spine may be considered34-36). However, the results of surgical treatment of FBSS may be unsatisfactory37-39). In our patients with severe LBP, SCNEN treatment yielded pain improvement, suggesting that SCNEN treatment may be an option to address persistent or new LBP after fusion surgery.
Limitation
Our study has some limitations. The number of patients was small, and the postoperative follow-up period was relatively short (mean, 28 months). To assess the eventual rate of LBP recurrence resulting from scar formation or nerve adhesion, long-term follow-up studies are needed. As all patients included in this study had undergone fusion surgery at another hospital, we do not know whether they had SCNEN before the surgery. Of the 30 patients subsequently admitted to our hospital, 8 (26.6%) were diagnosed with SCNEN. In earlier reports, this rate was 1.6%-14%25,27). We do not know whether this difference is due to background differences or whether the incidence of SCNEN is high after LFS. SCNEN treatment is not common and may not be performed at the institution where patients with LBP are first seen. Further studies on the incidence of SCNEN in patients reporting LBP are needed.
Conclusion
We report the successful treatment of postoperative LBP attributable to SCNEN in 8 patients who had undergone LFS. SCN blocks and SCN neurolysis under local anesthesia may improve LBP in such patients. Our observations suggest that SCNEN is a possible source of persistent or new LBP after LFS and that its treatment may be useful in patients with FBSS after LFS.
Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Maigne JY, Planchon CA. Sacroiliac joint pain after lumbar fusion. A study with anesthetic blocks. Eur Spine J. 2005;14(7): 654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshihara H. Sacroiliac joint pain after lumbar/lumbosacral fusion: current knowledge. Eur Spine J. 2012;21(9):1788-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose B. Outcomes after posterolateral lumbar fusion with instrumentation in patients treated with adjunctive pulsed electromagnetic field stimulation. Adv Ther. 2001;18(1):12-20. [DOI] [PubMed] [Google Scholar]
- 4.Kim K, Isu T, Chiba Y, et al. The usefulness of ICG video angiography in the surgical treatment of superior cluneal nerve entrapment neuropathy. J Neurosurg Spine. 2013;19(5):624-8. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto D, Isu T, Kim K, et al. Surgical treatment of superior cluneal nerve entrapment neuropathy. J Neurosurg Spine. 2013;19(1):71-5. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex and size. A prospective controlled clinical study. Spine. 1994;19(14):1611-8. [DOI] [PubMed] [Google Scholar]
- 7.Jang JS, Lee SH, Min JH, et al. Changes in sagittal alignment after restoration of lower lumbar lordosis in patients with degenerative flat back syndrome. J Neurosurg Spine. 2007;7(4):387-92. [DOI] [PubMed] [Google Scholar]
- 8.Legaye J, Duval-Beaupère G, Hecquet J, et al. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WJ, Lai PL, Niu CC, et al. Surgical treatment of adjacent instability after lumbar spine fusion. Spine. 2001;26(22):E519-24. [DOI] [PubMed] [Google Scholar]
- 10.Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine. 2004;29(17):1938-44. [DOI] [PubMed] [Google Scholar]
- 11.Fischgrund JS, MackayM, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22(24):2807-12. [DOI] [PubMed] [Google Scholar]
- 12.Tokuhashi Y, Nishimura T, Matsuzaki Y. Clinical results of more than 10 years after posterolateral fusion with pedicle screw fixation for degenerative lumbar spondylolisthesis. Spine Spinal Cord. 2004;17:185-92. [Google Scholar]
- 13.Tokuhashi Y, Matsuzaki H, Oda H, et al. Clinical course and significance of the clear zone around the pedicle screws in the lumbar degenerative disease. Spine. 2008;33(8):903-8. [DOI] [PubMed] [Google Scholar]
- 14.Fritzell P, Hagg O, Wessberg P, et al. Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26(33):2521-32. [DOI] [PubMed] [Google Scholar]
- 15.Phillips FM, Slosar PJ, Youssef JA, et al. Lumbar spine fusion for chronic low back pain due to degenerative disc disease. Spine. 2013;38(7):E409-22. [DOI] [PubMed] [Google Scholar]
- 16.Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine. 2009;34(10):1094-109. [DOI] [PubMed] [Google Scholar]
- 17.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32(7):816-23. [DOI] [PubMed] [Google Scholar]
- 18.Burton CV. Causes of failure of surgery on the lumbar spine: ten-year follow up. Mt Sinai J Med. 1991;58(2):183-7. [PubMed] [Google Scholar]
- 19.Long DM. Failed back surgery syndrome. Neurosurg Clin N Am. 1991;2(4):899-919. [PubMed] [Google Scholar]
- 20.Long M, Filtzer DL, Bendebba M, et al. Clinical features of failed back syndrome. J Neurosurg. 1988;69(1):61-71. [DOI] [PubMed] [Google Scholar]
- 21.Slipman CW, Shin CH, Patel RK, et al. Etiologies of failed back surgery syndrome. Pain Med. 2002;3(3):200-14. [DOI] [PubMed] [Google Scholar]
- 22.Waguespack A, Schofferman J, Slosar P, et al. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002;3(1):18-22. [DOI] [PubMed] [Google Scholar]
- 23.Kuniya H, Aota Y, Kawai T, et al. Prospective study of superior cluneal nerve disorder as a potential cause of low back pain and leg symptoms. J Orthpaed Surg Res. 2014;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuniya H, Aota Y, Saito T, et al. Anatomical study of superior cluneal nerve entrapment. J Neurosurg Spine. 2013;19(1):76-80. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Ebraheim NA, Huntoon M, et al. Anatomic considerations of superior cluneal nerve at posterior iliac crest region. Clin Orthop Relat Res. 1998;(347):224-8. [PubMed] [Google Scholar]
- 26.Akbas M, Yegin A, Karsli B. Superior cluneal nerve entrapment eight years after decubitus surgery. Pain Pract. 2005;5(4):364-6. [DOI] [PubMed] [Google Scholar]
- 27.Maigne JY, Doursounian L. Entrapment neuropathy of the medial superior cluneal nerve. Nineteen cases surgically treated, with a minimum of 2 years' follow-up. Spine. 1997;22(10):1156-9. [DOI] [PubMed] [Google Scholar]
- 28.Kuniya H, Aota Y, Nakamura N, et al. [Low back pain patients with suspected entrapment of the superior cluneal nerve.] J Spine Res. 2011;2(6):1032-5. Japanese [Google Scholar]
- 29.Talu GK, Ozyalcin S, Talu U. Superior cluneal nerve entrapment. Reg Anesth Pain Med. 2000;25(6):648-50. [DOI] [PubMed] [Google Scholar]
- 30.Ahlmann E, Patzakis M, Roidis N, et al. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surgery. 2002;84(5):716-20. [DOI] [PubMed] [Google Scholar]
- 31.Baumhauer J, Pinzur MS, Donahue R, et al. Site selection and pain outcome after autologous bone graft harvest. Foot Ankle Int. 2014;35(2):104-7. [DOI] [PubMed] [Google Scholar]
- 32.Hartwig T, Streiparth F, Grob C, et al. Digital 3-dimensional analysis of the paravertebral lumbar muscles after circumferential single-level fusion. J Spinal Disord Tech. 2011;24(7):451-4. [DOI] [PubMed] [Google Scholar]
- 33.Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20(7):753-8. [PubMed] [Google Scholar]
- 34.Assaker R, Zairi F. Failed back surgery syndrome: to re-operate or not to re-operate? A retrospective review of patient selection and failures. Neurochirurgie. 2015;61(1):S77-82. [DOI] [PubMed] [Google Scholar]
- 35.Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29(7):726-33. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, Yagi M, Machida M, et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J. 2015;15(7):1536-44. [DOI] [PubMed] [Google Scholar]
- 37.Chang MS, Chang YH, Revella J, et al. Revision spinal fusion in patients older than 75. Is it worth the risks? Spine. 2013;39(1):E35-9. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine. 2004;30(1):152-60. [DOI] [PubMed] [Google Scholar]
- 39.Van Buyten JP. Neurostimulation for chronic neuropathic back pain in failed back surgery syndrome. J Pain Symptom Manage. 2006;31(4):S25-9. [DOI] [PubMed] [Google Scholar]


