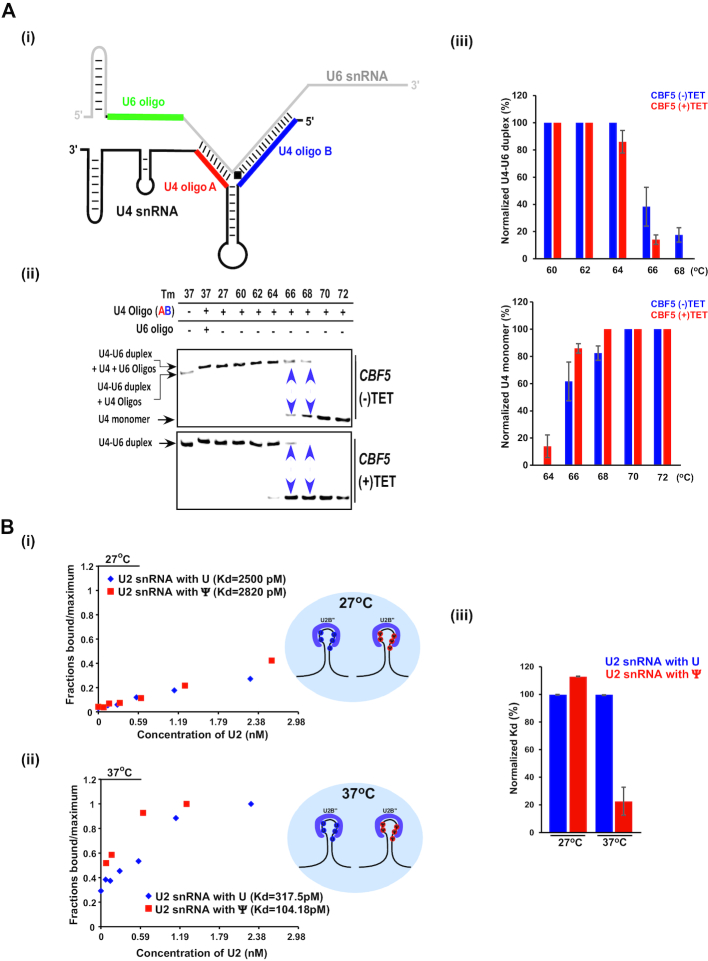
Figure 7.
Ψs regulate RNA and protein interactions at elevated temperature. (A) The stability of the U4/U6 duplex depends on its Ψs. (i) Schematic representation showing the position of the oligonucleotides used for examining the dimeric U4/U6 complex. (ii) U4/U6 duplexes were reconstituted from total RNA derived from cells carrying a CBF5 silencing construct, either uninduced (-TET) or after 2.5 days of silencing (+TET). Complexes were incubated with the indicated oligonucleotide, and incubated at different temperatures as indicated. Annealed RNA was separated on a 12% native gel and subjected to Northern analysis with a U4 RNA probe. The purple arrowheads represent the dissociation of the U4/U6 duplex. (iii) Percentage of U4/U6 duplex and U4 monomer was calculated from three independent biological replicates (as shown in Supplementary Figure S10B) using ImageJ software (https://imagej.nih.gov/ij/). Data are presented as mean ± S.E.M. (B) A dose response of Cy5 labeled U2 snRNA binding to U2B'' protein in a microfluidic device. After the U2B'' protein-U2 snRNA interaction reached equilibrium at either 27°C (i) or 37°C (ii), the free and U2B'' bound RNA concentration was measured. The data were normalized to maximum, and affinity was calculated by non-linear least squares fitting. Representative graph of three independent replicates is presented. (iii) Normalized Kd was calculated for the interaction of U2B" protein with U2 snRNA carrying either Ψ or U. Data are presented as mean ± S.E.M. Experiments were done in triplicate (n = 3).