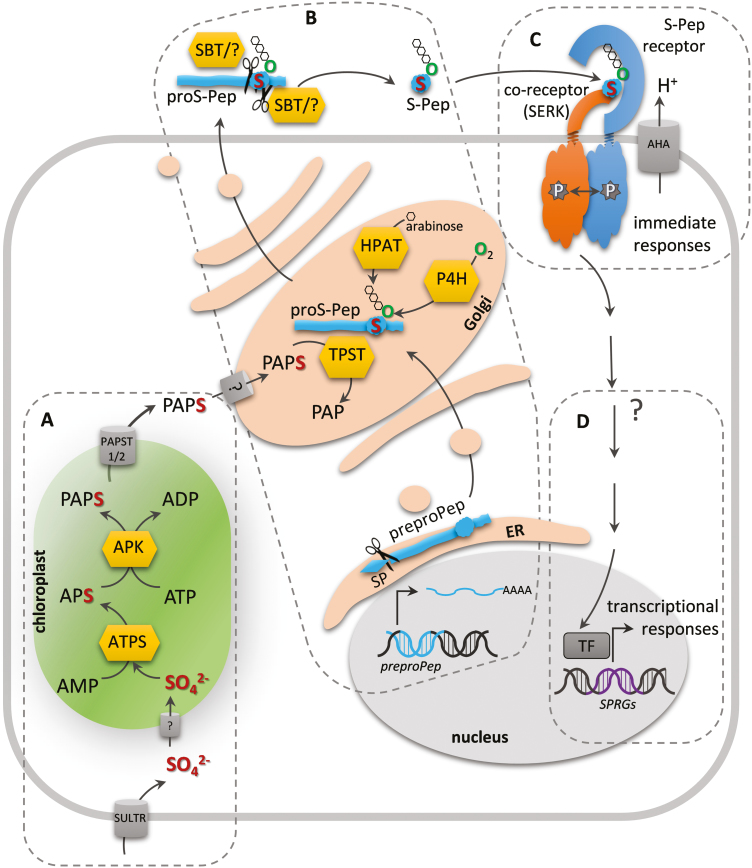
Fig. 1.
Activation of sulfate, maturation of sulfated peptides, and peptide perception. (A) Sulfate uptake occurs via sulfate transporters (SULTR) into the cytoplasm, but the transporter into the chloroplast is unknown. ATP sulfurylase (ATPS) and APS kinase (APK) produce the tyrosylprotein sulfotransferase (TPST) co-substrate 3´-phosphoadenosine 5´-phosphosulfate (PAPS) (Mugford et al., 2009). The PAPS transporter (PAPST) exports it into the cytoplasm, but how PAPS enters the Golgi is not known (Gigolashvili et al., 2012). (B) Maturation of sulfated peptides includes cleavage of the signal peptide (SP) in the ER during preproprotein synthesis, tyrosine sulfation by TPST in the Golgi (Komori et al., 2009), proline hydroxylation by prolyl-4-hydroxylase (P4H) and triarabinosylation by hydroxyproline O-arabinosyltransferase (HPAT) in the case of PSYs in the Golgi (Amano et al., 2007), and cleavage of the N- and C-terminals by subtilases (SBT) and/or other unknown proteases probably in the apoplast to release the mature sulfated peptide (S-Pep) (Srivastava et al., 2008; Ghorbani et al., 2016). (C) PSKR, PSY1R, and RGFRs are perceived at the plasma membrane by a receptor/co-receptor pair that mutually transphosphorylate each other and activate the receptor (Hohmann et al., 2017). The proton-pumping H+-ATPase (AHA) has been identified as a direct target of the S-Pep receptors PSKR1 and PSY1R (Fuglsang et al., 2014; Ladwig et al., 2015) (D) S-Peps are predicted to regulate S-Pep-responsive genes (SPRGs) via unknown signaling intermediates and transcription factors (TF).