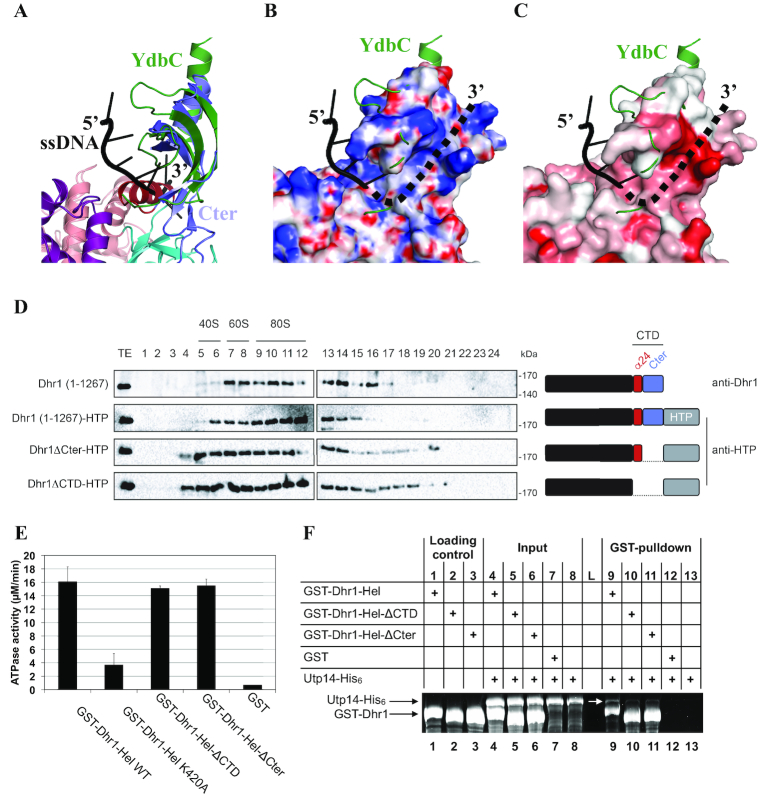
Figure 3.
The CTD of Dhr1 is required for its recruitment to precursor ribosomes in vivo and for Utp14 binding in vitro. (A) Superimposition of YdbC (green) bound to ssDNA (black) onto Dhr1 CTD. (B) Mapping of the electrostatic potential (colored from –15 kBT/e− in red to 15 kBT/e− in blue) on the surface of the Dhr1 CTD. In panels B and C, the YdbC–ssDNA complex is represented with the same color code and in the same orientation as in A. The proposed path for the 3′ ssRNA extremity is shown as a dotted line. The electrostatic potential was calculated using the PBEQ Solver server (62). (C) Mapping of the sequence conservation at the surface of the Dhr1 CTD. Coloring is from grey (no conservation) to red (high conservation). The conservation score was calculated using the Consurf server from a sequence alignment of 45 fungal Dhr1 (63). (D) Velocity gradient analysis of strains expressing various Dhr1 constructs. Detection with anti-Dhr1 antibody in diploid BY4743 cells (first lane), and detection with anti-HTP in heterozygous DHR1-HTP/DHR1 (other lanes). The Dhr1 constructs used in this experiment are schematically depicted on the right of the sucrose gradient profiles. This experiment was repeated three times, a representative example is shown. (E) The CTD of Dhr1 has no significant influence on its RNA-dependent ATPase activity. Identical amounts of the indicated recombinant constructs were engaged in a classical green malachite ATPase assay ((64) and see Materials and Methods). The experiment was reproduced three times independently (the mean values and s.d. are shown). Note that poly-(A) RNA was used in these experiments. (F) The CTD of Dhr1 is enhancing the interaction with its coactivator Utp14 in vitro. The interaction between various Dhr1-Hel constructs and Utp14 was addressed by GST-pulldown assays. Input and eluate (GST-pulldown) fractions were analyzed on 10% SDS-PAGE and stained with Sypro Ruby. L: Molecular weight ladder.