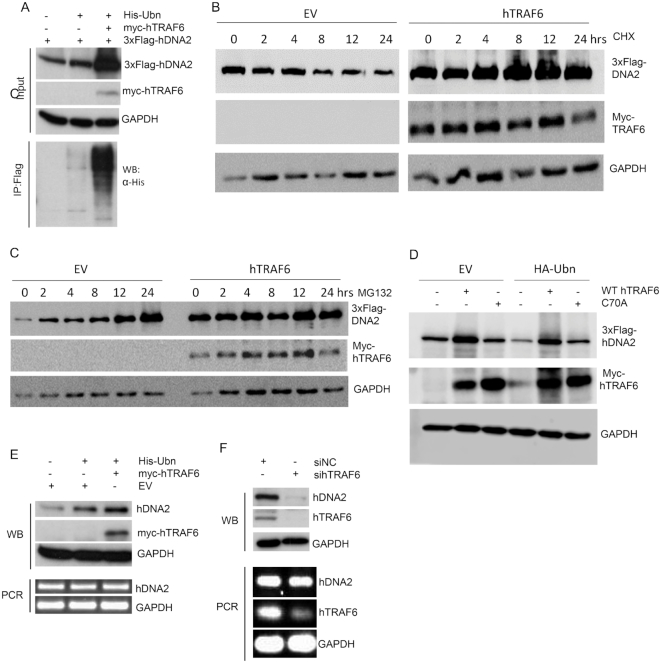
Figure 3.
hTRAF6-mediated hDNA2 ubiquitation is important for the stability of hDNA2. (A) 3xFlag-hDNA2 was expressed alone or with 6His-Ubn and/or myc-hTRAF6 in 293T cells. 3xFlag-hDNA2 was detected by western blot analysis using an anti-Flag antibody. GAPDH was used as an internal control. The ubiquitination status of 3xFlag-hDNA2 was analyzed as described for Figure 2D. (B and C) 293T cells were transfected with 3xFlag-hDNA2 and an empty control vector (EV) or myc-hTRAF6. The cells were treated with CHX (panel B, 50 μg/ml) or MG132 (Panel C, 1 μM) and harvested and lysed at 0, 2, 4, 8, 12 or 24 h. 3xFlag-hDNA2 levels were determined by western blot using anti-Flag antibody. GAPDH was used as a loading control. (D) 3xFlag-hDNA2 was co-expressed with WT or C70A mutant myc-hTRAF6 and with empty vector (EV) or the vector expressing HA-ubiquitin (HA-Ubn). In the controls, 3xFlag-hDNA2 expression vector was co-transfected with EV or HA-Ubn vector. 3xFlag-hDNA2 levels were determined by western blot using anti-Flag antibody. (E) 6His-Ubn and/or myc-hTRAF6 were overexpressed in HeLa cells, and endogenous hDNA2 protein and mRNA levels were evaluated by western blot analysis and RT-PCR, respectively. HeLa cells harboring EV only were used as the control. GAPDH was used as an internal control for comparison of hDNA2 levels in different cells. (F) HeLa cells were transfected with a scrambled negative control siRNA (siNC) or siRNA against hTRAF6 (sihTRAF6). Endogenous hDNA2 protein and mRNA levels were evaluated by western blot analysis and RT-PCR, respectively. hTRAF6 knockdown efficacy was confirmed by RT-PCR. GAPDH was used as an internal control.