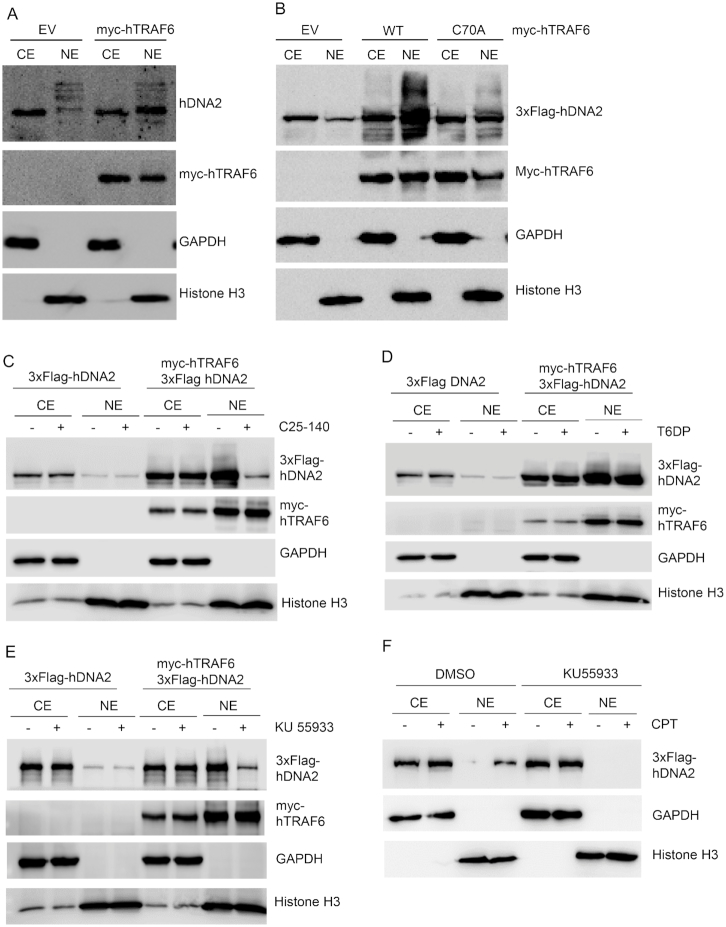
Figure 4.
hTRAF6 promotes hDNA2 nuclear localization. (A) HeLa cells were transfected with an empty control vector (EV) or a vector encoding myc-hTRAF6. Endogenous hDNA2 protein in the CE and NE was analyzed by western blot analysis using an anti-hDNA2 antibody. GAPDH and histone H3 were used as CE and NE markers, respectively. (B) 3xFlag-hDNA2 was co-expressed with EV or a vector encoding WT or C70A (ubiquitin E3 ligase activity-deficient) mutant hTRAF6 in 293T cells. 3xFlag-hDNA2 in the CE and NE was analyzed by western blot analysis using an anti-Flag antibody. GAPDH and histone H3 were used as CE and NE markers, respectively. (C–E) 293T cells co-expressing 3xFlag-hDNA2 and myc-hTRAF6 were left untreated or were treated with (C) a small molecule inhibitor of TRAF6 E3 ligase activity (C25–140, 30 μM, 48 h), (D) a TRAF6/RANK interaction-disrupting peptide (T6DP, 100 μM, 24 h) or (E) a small molecule ATM kinase activity inhibitor (KU-55933, 10 μM, 16 h). 3xFlag-hDNA2 levels in the CE and NE were analyzed by western blot analysis using an anti-Flag antibody. GAPDH and histone H3 were used as CE and NE markers, respectively. (F) 293T cells expressing 3xFlag-hDNA2 were pre-treated with DMSO (control) or KU-55933 (10 μM, 16 h). The cells were left untreated or were treated with CPT (1 μM, 4 h). 3xFlag-hDNA2 levels in the CE and NE were analyzed by western blot analysis using an anti-Flag antibody. GAPDH and histone H3 were used as CE and NE markers, respectively.